Sodium bromide, abbreviated NaBr, is an inorganic compound. It is an inorganic sodium salt with the counterion bromide. It is a white, crystalline solid with a high melting point that resembles sodium chloride. It contains a bromide salt as well as an inorganic sodium salt. It is a common source of bromide ion and has numerous applications.
This salt has numerous applications in the chemistry and pharmaceutical industries. Its solubility prevents it from occurring naturally in rocks or naturally occurring minerals. It has no odor and a slightly bitter and briny taste, but it is extremely toxic.
Properties
It is a white crystal or white, granular powder having the odour of sulphur dioxide. It does not occur as a natural solid due to its solubility, it is extracted from ocean water along with chlorides, iodides and halites.
- Chemical formula: NaBr
- Molar mass: 102.894 g·mol−1
- Appearance: White powder, hygroscopic
- Density: 3.21 g/cm3 (anhydrous); 2.18 g/cm3 (dihydrate)
- Melting point: 747 °C (anhydrous), 36 °C (dihydrate) decomposes
- Boiling point: 1,390 °C (2,530 °F; 1,660 K)
- Solubility in water: 71.35 g/100 mL (−20°C); 79.52 g/100 mL (0°C), 116.2 g/100 mL (100°C)
- Solubility: Soluble in alcohol, liquid ammonia, pyridine, hydrazine, SO2
- Insoluble in: acetone, acetonitrile

Synthesis, structure, reactions
NaBr crystallizes in the same cubic motif as NaCl, NaF and NaI. The anhydrous salt crystallizes above 50.7 °C. Dihydrate salts (NaBr·2H2O) crystallize out of water solution below 50.7 °C. NaBr is produced by treating sodium hydroxide with hydrogen bromide.
Sodium bromide can be used as a source of the chemical element bromine. This can be accomplished by treating an aqueous solution of NaBr with chlorine gas:
2 NaBr + Cl → Br2 + 2 NaCl
Preparation of other bromine compounds
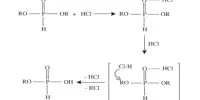
Sodium bromide is widely used for the preparation of other bromides in organic synthesis and other areas. It is a source of the bromide nucleophile to convert alkyl chlorides to more reactive alkyl bromides by the Finkelstein reaction:
NaBr + RCl → RBr + NaCl (R = alkyl)
The photosensitive salt silver bromide is prepared using NaBr, which was once in high demand in photography but is now in short supply.
Because of its high water solubility (943.2 g/L or 9.16 mol/L at 25 °C), sodium bromide is used to prepare dense drilling fluids used in oil wells to compensate for potential overpressure in the fluid column and to counteract the associated tendency to blow out. The presence of sodium cation causes the bentonite in the drilling fluid to swell, while the high ionic strength causes the bentonite to flocculate.
Applications
In industry, sodium bromide is the most useful inorganic bromide. It is also used in TEMPO-mediated oxidation reactions as a catalyst. It is used to disinfect hot tubs and swimming pools in conjunction with chlorine.
- Like other bromides, it is used as a sedative.
- The oil and gas drilling industry is a major consumer of sodium bromide.
- Used for germicidal properties due to bromine liberation.
- Used in pharmaceutical preparations as an antiseptic, detergent, and reagent.
Safety
NaBr is extremely toxic, with an oral LD50 of 3.5 g/kg for rats. This, however, is a single-dose value. Bromide ion is a cumulative toxin with a relatively long half life (in humans, more than a week): see potassium bromide.