Hydrogen halides (hydrohalic acids in the aqueous phase) are diatomic, inorganic compounds that act as Arrhenius acids in chemistry. The formula is HX, where X can be fluorine, chlorine, bromine, iodine, or astatine. At Standard Temperature and Pressure, all known hydrogen halides are gases. Hydrogen fluoride (HF), hydrogen chloride (HCl), hydrogen bromide (HBr), and hydrogen iodide (HI) are examples of common hydrogen halides.
Physical properties
Except for hydrogen fluoride, which boils at 19 °C, the hydrogen halides are colourless gases at standard temperature and pressure (STP). Because hydrogen fluoride is the only hydrogen halide that exhibits hydrogen bonding between molecules, it has the highest melting and boiling points of the HX series.
- Physical State: Except for hydrogen iodide, which is a reddish-brown gas at room temperature, these gases are colourless at room temperature. Because of its stronger intermolecular forces, hydrogen fluoride is a colourless liquid.
- Solubility: These are extremely water soluble. When hydrofluoric acid (HF), hydrochloric acid (HCl), hydrobromic acid (HBr), and hydroiodic acid (HI) are dissolved in water, they form strong acids known as hydrohalic acids.
- Acidity: Because of the high electronegativity of the halogen atoms, these are strong acids. They completely dissociate in water, releasing hydrogen ions (H+) and halide ions (F-, Cl-, Br-, I-), raising the concentration of H+ ions and decreasing the pH.
- Boiling Points: The boiling points of hydrogen halides rise as the atomic number of the halogen increases. This is due to the increasing strength of the van der Waals forces between the molecules as the halogen atoms’ size and polarizability increase. Among the hydrogen halides, hydrogen fluoride has the highest boiling point.
- Reactivity: These are highly reactive and can go through a variety of chemical reactions. Depending on the reaction conditions, they can react with metals, metal oxides, metal hydroxides, and carbonates to form halides and water or carbon dioxide.
The boiling point increases from HCl to HI. The increasing strength of intermolecular van der Waals forces, which correlates with the number of electrons in the molecules, is attributed to this trend. Hydrohalic acid solutions that have been concentrated emit visible white fumes. This mist is produced by the formation of tiny droplets of their concentrated aqueous hydrohalic acid solutions.
Synthesis
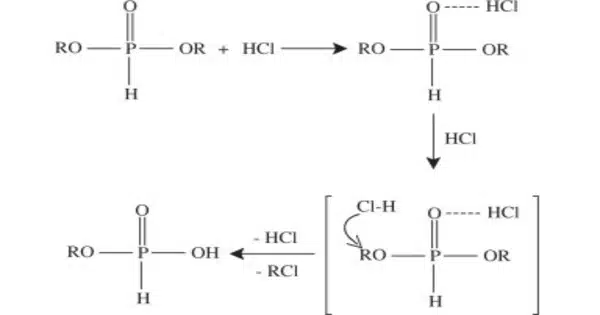
Hydrogen fluoride and hydrogen chloride are produced by direct reactions of hydrogen with fluorine and chlorine, respectively. However, these gases are produced industrially by treating halide salts with sulfuric acid. When hydrogen and bromine are combined at high temperatures in the presence of a platinum catalyst, hydrogen bromide is formed. The least stable hydrogen halide, HI, is created indirectly through the reaction of iodine with hydrogen sulphide or hydrazine.
Occurrence
Hydrogen chloride, in the form of hydrochloric acid, is a major component of gastric acid.
Hydrogen fluoride, chloride and bromide are also volcanic gases.
Toxicity
Hydrogen halides can be toxic, especially when inhaled in high concentrations. They are corrosive to the skin, eyes, and respiratory system. Precautions should be taken when handling these compounds.