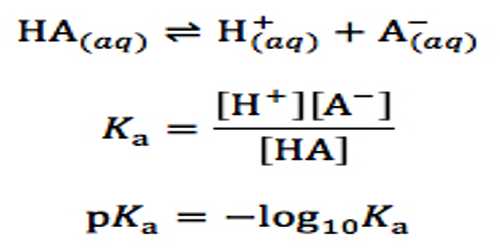pH of solutions of very weak acids
If the acid is very weak then the extent of dissociation will be very small and for all practical purposes [HA] in the equation may be taken as the initial concentration of the acid. As [H+] = [A–] equation reduces to
[H+]2 = Ka [HA]
so that, [H+] = √{Ka [HA]} … … … (1)
This relation may be used to make approximate calculations of pH of solutions of very weak and moderately weak acids. If the pH of the solution of a weak acid is known an approximate value of its Ka may be calculated. The value of Ka may also be determined by a titration curve.
Example: Calculate the pH of a solution of n-butyric acid concentration 0.12 mol L-1 (Ka of the acid = 1.515 x 10-5 mol L-1).
Solution: Using the relation (1), [H+] = √{Ka [HA]}
= √[0.12×1.515×10-5]
= 1.66 x 10-3
pH = – log (1.66 x 10-3) = 2.78.
pH is a measure of the concentration of hydrogen ions in a solution. Strong acids like hydrochloric acid at the sort of concentrations you normally use in the lab have a pH around 0 to 1. The lower the pH, the higher the concentration of hydrogen ions in the solution./sub