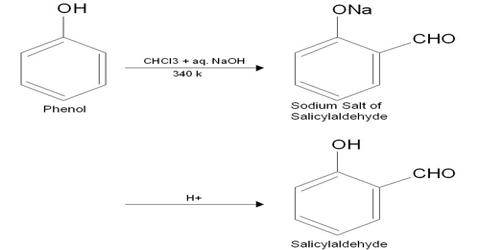
Reimer-Tiemann reaction: In presence of NaOH, Chloroform reacts with phenol at 60°-70°C to produce salicylaldehyde, this reaction is known as Reimer- Tiemann reaction. The mechanism begins with the abstraction of the proton from chloroform with the base to form a trichlorocarban ion which spontaneously loses a chloride ion to form a neutral dichlorocarbene.
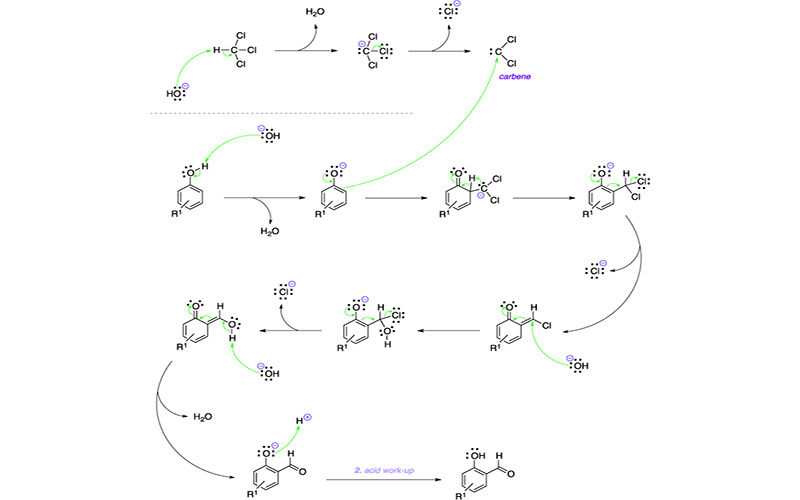
The Reimer-Tiemann reaction is an organic reaction used to convert a phenol to an o-hydroxy benzaldehyde using chloroform, a base, and acid work-up. Dichlorocarbenes can also react with alkenes and amines to form dichlorocyclopropanes and isocyanides, respectively. As such the Reimer-Tiemann reaction may be unsuitable for substrates bearing these functional groups. In addition, many compounds cannot withstand being heated in the presence of hydroxide.