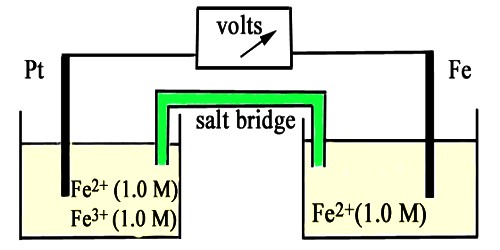
Oxidation-reduction Electrode in Half-Cells
A half cell is one of the two electrodes in a galvanic cell or simple battery. For example, in the Zn-Cu battery, the two half cells make an oxidizing-reducing couple.
This name is generally used for electrodes in which an inert metal dips into a solution containing ions of the same element in two different oxidation states. Oxidation reduction is a type of chemical reaction in which electrons are transferred from one substance to another. The oxidized species loses electrons and the reduced species gains electrons. An example is the half-cell Pt/Fe2+, Fe3+ in which the following reaction takes place-
Fe3+ (aq) + e– ↔ Fe2+ (aq)
It will be recalled that in all electrodes either oxidation or reduction takes place. The difference between an oxidation-reduction electrode and other electrodes, e.g., a Ag│Ag+ electrode, is that whereas in the latter the e.m.f depends only on the concentration of the silver ions, in the former the e.m.f. is dependent on the concentration of the ion in both the oxidation states.