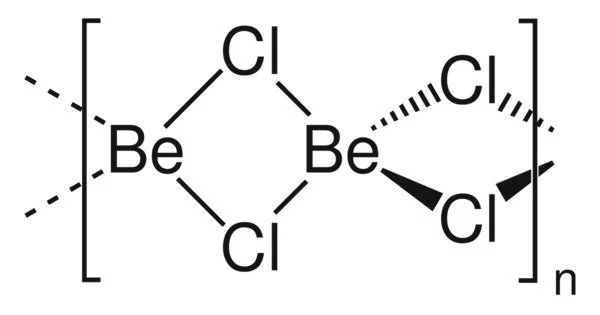
Beryllium chloride, also known as BeCl2, is an inorganic compound. It is a colorless, hygroscopic solid that dissolves readily in a wide range of polar solvents. Because of beryllium’s diagonal relationship with aluminum, its properties are similar to those of aluminium chloride.
Beryllium chloride is produced by a high-temperature reaction of the metal with chlorine. It can also be synthesized through carbothermal reduction of beryllium oxide in the presence of chlorine. It is made by reacting Be metal with hydrogen chloride.
Properties
Beryllium Chloride is a colourless, hygroscopic solid that dissolves well in many polar solvents. It is known as an electron-deficient compound because it has the two empty orbitals at the bonding level. It reacts vigorously and exothermically with water with the evolution of acidic, steamy hydrogen chloride gas.
- Chemical formula: BeCl2
- Molar mass: 79.9182 g/mol
- Appearance: White or yellow crystals
- Density: 1.899 g/cm3, solid
- Melting point: 399 °C (750 °F; 672 K)
- Boiling point: 482 °C (900 °F; 755 K)
- Solubility in water: 15.1 g/100 mL (20 °C)
- Solubility: soluble in alcohol, ether, benzene, and pyridine, slightly soluble in chloroform and sulfur dioxide
- Crystal structure: hexagonal
- Molecular shape: polymer
Structure and synthesis
Beryllium chloride is prepared by reaction of the metal with chlorine at high temperatures:
Be + Cl2 → BeCl2
BeCl2 can also be synthesized through the carbothermal reduction of beryllium oxide in the presence of chlorine. BeCl2 can be made by combining beryllium and hydrogen chloride.
BeCl2 exists in two forms (polymorphs). Both structures are made up of tetrahedral Be2+ centers linked together by doubly bridging chloride ligands. Edge-sharing polytetrahedra are one form. The other form has interconnected adamantane-like cages that resemble zinc iodide. BeF2, on the other hand, is a three-dimensional polymer with a structure similar to quartz.
In the gas phase, BeCl2 exists both as a linear monomer and a bridged dimer with two bridging chlorine atoms where the beryllium atom is 3-coordinate. The linear shape of the monomeric form is as predicted by VSEPR theory. The linear shape contrasts with the monomeric forms of some of the dihalides of the heavier members of group 2, e.g. CaF2, SrF2, BaF2, SrCl2, BaCl2, BaBr2, and BaI2, which are all non-linear.
Prepareration
It is synthesized by the reaction of beryllium with chlorine at a temperature of about 250 °C, which is represented by the following equation:
Be + Cl2 → BeCl2
When beryllium oxide reacts with chlorine in the presence of carbon at a temperature of about 700-900 °C, it undergoes carbothermal reduction to yield carbon monoxide and beryllium chloride :
BeO + Cl2 + C → BeCl2 + CO
BeCl2 can also be produced by the reaction of beryllium with a diluted solution of hydrogen chloride:
Be + 2HCl → BeCl2 + H2
Reactions
Beryllium chloride forms a tetrahydrate, BeCl2•4H2O ([Be(H2O)4]Cl2). BeCl2 is also soluble in some ethers.
Applications
Beryllium chloride is used as a raw material in beryllium electrolysis and as a catalyst in Friedel-Crafts reactions.
- It acts as a catalyst as well as a chemical intermediate in the synthesis of several beryllium compounds.
- It is used as a raw material in the electrolytic extraction of beryllium.
Safety
Beryllium chloride is extremely toxic if inhaled, swallowed, or applied to the skin. It causes skin corrosion, allergic skin reactions, and irritation of the eyes and respiratory tract. It is known to be a carcinogen in humans and, when exposed repeatedly, can cause organ toxicity, including lung cancer.