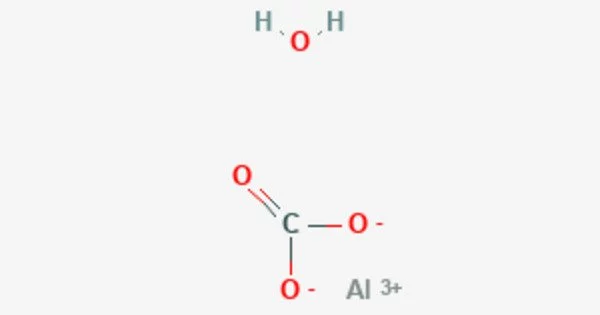
Aluminum carbonate is a chemical compound with the formula Al2(CO3)3. It is a white, crystalline powder that is used in a variety of industrial and commercial applications, such as water treatment, ceramics, and catalysts. It can also be used as an antacid in medicine. It is formed by the reaction of aluminum hydroxide with carbon dioxide.
It is an aluminum carbonate. It is not well characterized; according to one authority, simple carbonates of aluminum are unknown. However, related compounds such as the basic sodium aluminum carbonate mineral dawsonite [NaAlCO3(OH)2] and the hydrated basic aluminum carbonate minerals scarbroite (Al5(CO3)(OH)13•5(H2O)) and hydroscarbroite (Al14(CO3)3(OH)36•nH2O) are known.
Properties
It is a white, crystalline solid that is insoluble in water. It is typically produced by the reaction of aluminum hydroxide with carbon dioxide. It is used in various industrial applications such as in the production of aluminum metal, as a filler in the paper industry, and as a component in ceramics and glass.
- Chemical formula: Al2(CO3)3
- Appearance: white powder, unstable
- Density: 1.5 g/cm3
- Melting point: 58 °C
- Boiling point: decomposes
- Solubility in water: reacts

Preparation
There is no evidence that aluminium carbonate is formed in double displacement reactions; soluble carbonates are alkaline enough to precipitate aluminium hydroxide and generate carbon dioxide. Although aluminium carbonate is highly unstable, when exposed to CO2, carbonate species readily form on the surface of aluminium oxide.
Aluminium carbonate is not naturally present in nature as they are highly unstable. There are two reported methods of production of aluminium carbonate. The first one encompasses the production of the compound in a high-pressure environment in the presence of carbon dioxide, in this furnace the temp is maintained near 0°C.
It can be manufactured with the high pressure of carbon dioxide and temperature close to 0°C. For aluminium carbonate storage, one would have to create a very complex system that would protect the substance against any external factors.
Uses
Aluminium carbonate, along with aluminum hydroxide and aluminum oxide, is a phosphate-binding drug that is sometimes given to dogs and cats to bind intestinal phosphate and prevent dietary phosphate absorption as well as to decrease absorption of pancreatic phosphate. It is rarely used in humans due to toxicity concerns, but cats and dogs do not appear to have a toxic response to its presence.
- Used as a phosphate-binding drug that is administered to cats and dogs
- Used as a drug to treat stomach inflammation and ulcerations
- It is taken to prevent the formation of urinary stones in humans.