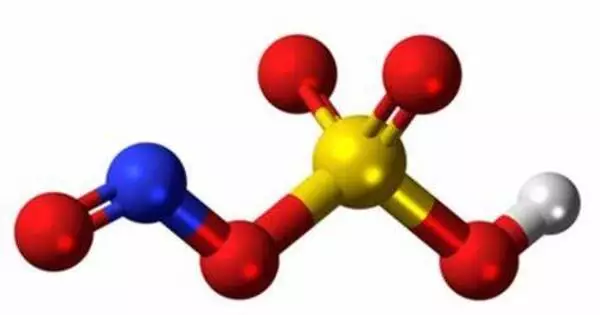
Nitrosylsulfuric acid is a chemical compound having the formula HSO4NO. It is a caustic and highly reactive chemical compound. It is a colorless solid that is utilized in the industrial manufacturing of caprolactam and was previously employed in the lead chamber process for the synthesis of sulfuric acid. The chemical is a combined anhydride of sulfuric acid and nitrous acid.
In organic chemistry, it is employed as a nitrosating reagent, a diazotizing agent, and an oxidizing agent.
Properties
It is a pale yellow to reddish-brown, oily liquid that emits toxic fumes when exposed to air. It is known for its extreme reactivity and is commonly used as a strong acid catalyst in various chemical processes.
- Chemical formula: HSO4NO
- Molar mass: 127.08 g/mol
- Appearance: Pale yellow crystals
- Density: 1.865 g/mL in 40% sulfuric acid soln
- Melting point: 70 °C (158 °F; 343 K)
- Boiling point: Decomposes
- Solubility in water: Decomposes
- Solubility: Soluble in H2SO4
Applications
- Sulfonation Reactions: It is often used in the sulfonation of organic compounds, a chemical reaction that introduces sulfonic acid groups (-SO3H) into organic molecules. This is important in the production of detergents, dyes, and pharmaceuticals.
- Dehydration Reactions: It can be used to remove water from various organic compounds, making it useful in dehydration reactions.
- Nitrating Agent: It is sometimes used as a nitrating agent to introduce nitro groups (-NO2) into organic molecules. This is a common step in the synthesis of explosives and some pharmaceuticals.
- Desulfonation Reactions: It can be used to remove sulfonic acid groups from organic compounds.
- Electroplating: It is used in electroplating processes as an electrolyte.
Safety
Nitrosylsulfuric acid is a dangerous substance, and extreme caution is advised. It must be handled with considerable caution due to its strong reactivity and caustic character. When it comes into touch with the skin, it can inflict serious burns and severe eye damage. Inhaling its vapors is also risky. When working with nitrosylsulfuric acid, safety equipment and correct handling practices are required. It should only be used in controlled laboratory or industrial environments by trained individuals.