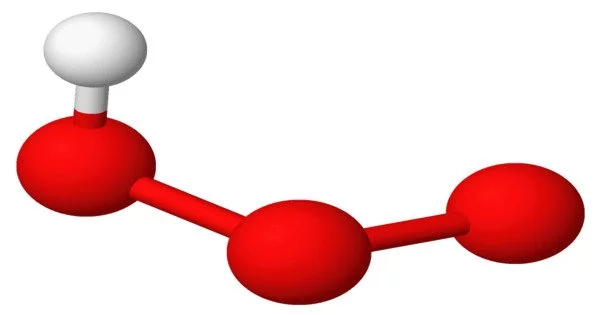
The hypothetical compound hydrogen ozonide (HOOH) is made up of hydrogen and ozonide ions. However, it is important to note that under normal conditions, hydrogen ozonide has not been observed or synthesised.
It is a radical molecule made up of a hydrogen atom that is covalently bonded to an ozonide unit. It could be produced by the hydroxyl radical reacting with dioxygen: OH• + O2 → HO3•. It was discovered in a mass spectrometer experiment that used HO+3 (protonated ozone) as a precursor.
When ozone (O3) accepts an electron, it produces a negatively charged species known as ozonide ions (O3-). Although hydrogen ozonide can theoretically form when an ozonide ion reacts with a hydrogen atom (H), the compound is highly unstable and prone to decomposition.
Properties
- Chemical Formula: HOOH
- Composition: It consists of hydrogen (H) and ozone (O3) molecules.
- Molecular Weight: The molecular weight is calculated as the sum of the atomic weights of its constituent elements, which gives a value of approximately 50.01 grams per mole.
- Stability: Ozonides, in general, tend to be highly unstable and can decompose explosively. Hydrogen ozonide is expected to be even more unstable due to the presence of reactive hydrogen atoms.
The instability of hydrogen ozonide arises from the high reactivity of ozonide ions. The addition of a hydrogen atom to an ozonide ion would create a compound with an unusual oxygen-oxygen-oxygen-hydrogen (O-O-O-H) arrangement. This arrangement is energetically unfavorable, making hydrogen ozonide highly reactive and likely to undergo rapid decomposition into more stable compounds.
Reactivity
Due to the presence of ozone, which is known for its strong oxidising properties, hydrogen ozonide is likely to be a powerful oxidising agent. It can have a strong reaction with reducing agents or flammable materials.
Because hydrogen ozonide contains hydrogen atoms, it is likely to react with water, potentially releasing oxygen gas (O2) and water (H2O).
Decomposition
Hydrogen ozonide would most likely decompose quickly, releasing molecular oxygen (O2) and hydrogen gas (H2), as well as possibly other reactive byproducts. The decomposition process could be highly exothermic, releasing a lot of energy.
Given its instability and potential for rapid decomposition, hydrogen ozonide could be highly explosive if large enough quantities were synthesised or formed.