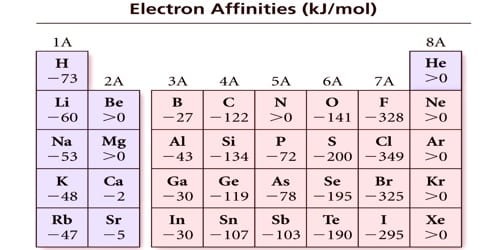
The electron affinity is defined as the energy transformation that occurs when an atom gains an electron, releasing energy in the procedure. So, it is defined as the transform in energy (in kJ/mole) of a neutral atom when an electron is added to the atom to form a negative ion.
Factors that affecting electron affinity –
(1) Atomic size
Electron affinity α [1/Size of the atom]
Smaller the size of an atom, greater is its electron affinity. As the size of the atom increases, the effective nuclear charge decreases or the nuclear attraction for adding electron decreases. Consequently, an atom will have less tendency to attract additional electron towards itself. Thus, smaller will be a force of attraction felt by incoming electron and therefore smaller will be the value of Electron affinity. Therefore, the electrostatic force of attraction will be more and the electron affinity will be higher. Its value increases as we move along a period since the size of atoms decreases along a period.
Therefore, Electron affinity α Effective nuclear charge. More the nuclear charge of the atom more stoutly will it magnetize additional electron. Greater the nuclear charge, greater will be the attraction for the incoming electron and as a consequence larger will be the value of electron affinity. In general, electron affinity decreases in going down the group and increases in going from left to right across the period. On moving down the group atomic size increases and on going from left to right in a period atomic size decreases. Therefore, electron-affinity increases as the nuclear charge increases. Thus, its value increases with an increase in nuclear charge along a period.
(2) Shielding or Screening Effect
Electron affinity α [1/Shielding effect]
The value of electron affinity increases with the reduction in the shielding effect of inner electrons. Electronic energy state, lying between the nucleus and outermost state hinder the nuclear attraction for incoming electron. Therefore, greater the number of inner lying stateless will be the electron affinity. The value of electron affinity also depends to some extent upon the category of orbital in which electron is added. The value is larger when electron enters ‘s’ orbital and decreases consecutively for p, d and f orbitals.
(3) Electronic Configuration –
The electronic configurations of elements influence their electron affinities to a considerable extent. Stable the configuration of an atom, smaller will be its propensity to accept an electron and therefore lower the value of its electron affinity.
Electron affinities of inert gases are zero. This is because their atoms have stable ns2 np6 configuration in their valence shell and there is no possibility for the addition of an extra electron. (example, those having completely filled or exactly half-filled outer orbitals).
Electron affinity of beryllium, magnesium, and calcium is practically zero. This is attributed to extra stability of the fully completed s-orbitals in them. Thus, if an atom has fully filled or half-filled orbitals, its electron affinity will be low.
For example, the elements having totally filled sub-levels of the valence shell have comparatively constant configurations and therefore, possess large positive values of electron affinity.