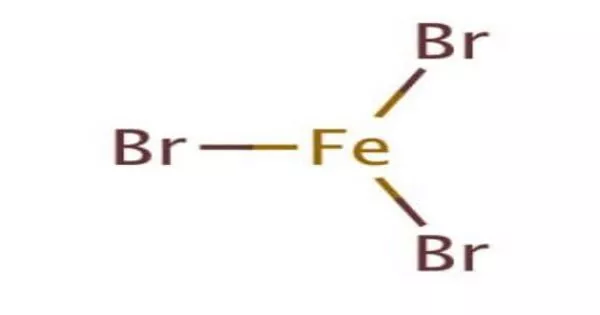
Iron(III) bromide is a chemical compound with the formula FeBr3. This red-brown, odorless compound, also known as ferric bromide, is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It dissolves in water to form acidic solutions.
Iron(III) bromide is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It is also used to make 2,5-dibromo-4-aryl-1,3-pentadiene by reacting with homoallenic bromohydrin.
Properties
Dark red hexagonal crystal; hygroscopic; partially decomposes to FeBr2, losing some bromine on exposure to air or light; density 4.50 g/cm3; decomposes on heating; soluble in water, ethanol, and ether.
- Chemical formula: FeBr3
- Molar mass: 295.56 g mol−1
- Appearance: brown solid
- Odor: odorless
- Density: 4.50 g cm−3
- Melting point: 200 °C (392 °F; 473 K) (decomposes)
- Crystal structure: Trigonal, hR24
Structure and synthesis
FeBr3 forms a polymeric structure featuring six-coordinate, octahedral Fe centers. Although inexpensively available commercially, FeBr3 can be prepared by treatment of iron metal with bromine:
2 Fe + 3 Br2 → 2 FeBr3
Above 200°C, FeBr3 decomposes to ferrous bromide:
2FeBr3 → 2FeBr2 + Br2
Iron(III) chloride is considerably more stable, reflecting the greater oxidizing power of chlorine. FeI3 is not stable, as iron(III) will oxidize iodide ions.
Preparation
Iron(III) bromide is prepared by the action of bromine with iron filings:
2Fe + 3Br2 → 2FeBr3
The compound should be stored in dark bottles protected from air or light. It also may be obtained by double decompostion reactions between a ferric salt and a bromide (alkali metal bromide) in aqueous solution followed by evaporation and crystallization:
Fe2(SO4)3 + LiBr → 2FeBr3 + 3Li2SO4
It also may be prepared in high yield by photochemical reaction of dibromoirontetracarbonyl with bromine in hexane.
(CO)4FeBr2 + ½ Br2 → FeBr3 + 4CO
Uses
Ferric bromide is used as an oxidant in organic chemistry on occasion, for example, in the conversion of alcohols to ketones. It is used as a Lewis acidic catalyst for aromatic compound bromination. It is frequently generated in situ for the latter applications.