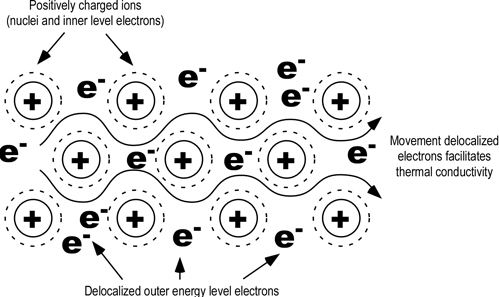
Because the electrons are free to move around the metal lattice, they can easily move through the metal when a potential difference is applied — i.e. an electric current can flow through the metal. The free electrons are the reason why solid metals are good electrical conductors. Thermal conductivity represents the quantity of energy transfered by surface unit and time unit , under a temperature gradient.
Thermal conductivity of Metals: When a metal is heated, the heat energy is transferred to the electrons. As these are delocalised, they can move easily throughout the metal, the heat is transferred easily as well. Thus metals are good thermal conductors.
Appearance of Metals: Light falling on metal surface can excite the electrons in the ‘sea into a higher energy level. When the electrons then return to a lower energy state, light is then re-emitted. This gives metals a shiny appearance.