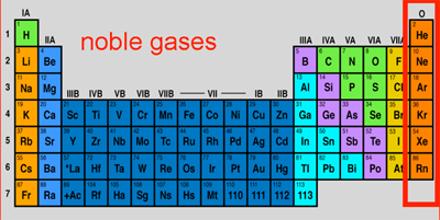
Noble gases are He, Ne, Ar, Kr, Xe, Rn. Helium has a filled shell of 2 electrons. All the rest have 8 electrons in their highest energy shells. The noble gases are either unreactive or of very low reactivity. They used to be called ‘inert gases’.
Bonding Only with the noble gases (Group 18 or 0) do elements occur as free atoms. Atoms of other elements occur in a combined form i.e. bonded to other atoms. Why do atoms combine?
- To go to a more stable electronic energy state, and
- Energy is normally given out or evolved when bonds are formed.
The stability of the Noble gases is attributed to an outer energy shell arrangement of eight electrons (except He, with two). When other atoms combine, they often behave as if they are seeking to achieve an outer octet of electrons – like the noble gases. An outer shell of eight electrons is an extremely stable electronic arrangement.
Octet rule or ‘rule of eight’
Atoms can achieve an outer shell of eight electrons by either:
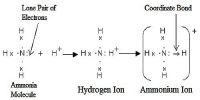
- Transferring electrons from one to another to produce ions. This is ionic bonding (or electrovalency), or
- Sharing electrons between one another.
This is called covalent bonding (or covalency).