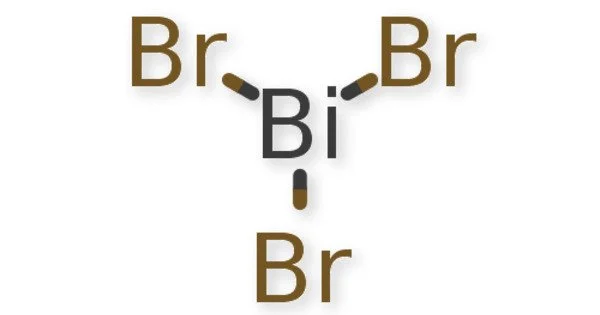
Bismuth tribromide is an inorganic compound of bismuth and bromine with the chemical formula BiBr3. It is a highly water soluble crystalline Bismuth source for uses compatible with Bromides and lower (acidic) pH. Most metal bromide compounds are water soluble for uses in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications.
Bismuth Tribromide is generally immediately available in most volumes. Ultra high purity and high purity compositions improve both optical quality and usefulness as scientific standards.
Properties
Bismuth trifluoride is a white powder, bismuth tribromide, BiBr 3, golden yellow crystals, bismuth iodide, Bi13, greyish-black crystals, are also known. It is a kind of yellow powder with a chemical formula of BiBr3 and a molecular weight of 448.692.
- Chemical formula: BiBr3
- Molar mass: 448.692 g·mol−1
- Appearance: white to light yellow or golden deliquescent crystals
- Density: 5.72 g/cm3 at 25 °C
- Melting point: 219 °C (426 °F; 492 K)
- Boiling point: 462 °C (864 °F; 735 K)
- Solubility in water: Soluble, slow hydrolysis
- Solubility: diethyl ether, THF

Preparation
It may be formed by the reaction of bismuth oxide and hydrobromic acid. It may be formed by the reaction of bismuth oxide and hydrobromic acid with the equation.
Bi2O3 + 6 HBr ⇌ 2 BiBr3 + 3 H2O
Bismuth tribromide can also be produced by the direct oxidation of bismuth in bromine.
2 Bi + 3 Br2 → 2 BiBr3
Structure
Bismuth tribromide adopts two different structures in the solid state: a low-temperature polymorph α-BiBr3 that is stable below 158 °C and a high-temperature polymorph β-BiBr3 that is stable above this temperature. Both polymorphs are monoclinic but α-BiBr3 is in space group P21/a whereas β-BiBr3 is in C2/m. α-BiBr3 consists of pyramidal molecules whereas β-BiBr3 is polymeric and adopts the AlCl3 structure. BiBr3 is the only group 15 trihalide that can adopt both molecular and polymeric structures.
Reactivity
Bismuth bromide is highly water-soluble. It is a Lewis acid and accepts bromide ions to form monomeric and oligomeric anionic complexes (bromobismuthates), e.g. [BiBr6]3−, [Bi2Br10]4−, (BiBr4-)n and (BiBr52-)n.
Similar phenomena are exhibited in the electrolysis of solutions of antimony tribromide and tri-iodide, the product obtained from the tribromide having a specific gravity of 5.4, and containing 18-20% of antimony tribromide, whilst that from the tri-iodide has a specific gravity of 5.2-5.8 and contains about 22% of hydriodic acid and antimony tri-iodide.