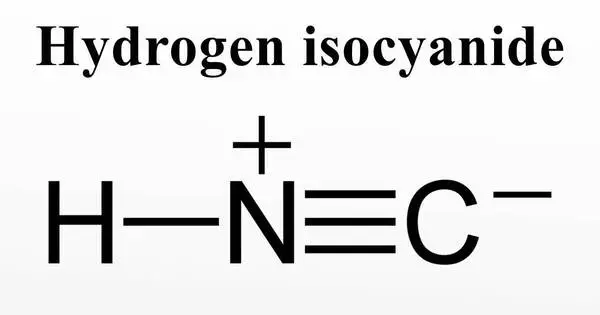Hydrogen isocyanide is a chemical with the molecular formula HNC. It is a colorless, volatile liquid with a pungent odor that is also known as hydrogen cyanide or HCN. It is a minor tautomer of hydrogen cyanide (HCN). It is made up of covalently linked hydrogen atoms (H), carbon atoms (C), and nitrogen atoms (N). Its importance in the field of astrochemistry is linked to its ubiquity in the interstellar medium. The chemical formula for hydrogen isocyanide is HCN.
HCN is extremely toxic and flammable. It can be found in small amounts in nature, such as in certain plants and fruits, but it is primarily produced industrially for a variety of purposes.
Nomenclature
Both hydrogen isocyanide and azanylidyniummethanide are correct IUPAC names for HNC. There is no preferred IUPAC name. The second one is according to the substitutive nomenclature rules, derived from the parent hydride azane (NH3) and the anion methanide (CH−3).
Properties
- Chemical formula: HNC
- Molar mass: 27.03 g/mol
- Conjugate acid: Hydrocyanonium
- Conjugate base: Cyanide
Molecular properties
Hydrogen isocyanide (HNC) is a linear triatomic molecule with C∞v point group symmetry. It is a zwitterion and an isomer of hydrogen cyanide (HCN). Both HNC and HCN have large, similar dipole moments, with μHNC = 3.05 Debye and μHCN = 2.98 Debye respectively. These large dipole moments facilitate the easy observation of these species in the interstellar medium.
Chemical Reactions
Hydrogen isocyanide can undergo various chemical reactions. It can polymerize under certain conditions, forming a solid polymer called polymeric isocyanide. It can also participate in addition reactions with other compounds, such as alkenes and aldehydes.
Applications
(1) Industrial Applications: Hydrogen isocyanide has several industrial applications, including:
- Production of synthetic fibers like nylon.
- Manufacturing of various plastics, including polyurethane.
- Extraction of precious metals, such as gold and silver, from ores.
- Production of certain pharmaceuticals and chemicals.
(2) Toxicity: HCN is extremely toxic to humans and many other animals. Inhalation of its vapors can be lethal at high concentrations. It interferes with cellular respiration by inhibiting the function of cytochrome c oxidase, an enzyme involved in the electron transport chain. This disruption prevents cells from utilizing oxygen, leading to tissue damage and eventual death.
Safety Precautions
Because of its toxicity, hydrogen isocyanide should be handled or worked with with extreme caution. Proper ventilation, personal protective equipment, and following safety protocols are required.
Cyanide poisoning can result from accidental or intentional ingestion of hydrogen isocyanide. Headache, dizziness, nausea, vomiting, rapid breathing, and confusion are some of the symptoms. It can result in loss of consciousness, cardiac arrest, and death in severe cases. In cases of cyanide poisoning, immediate medical attention is required.