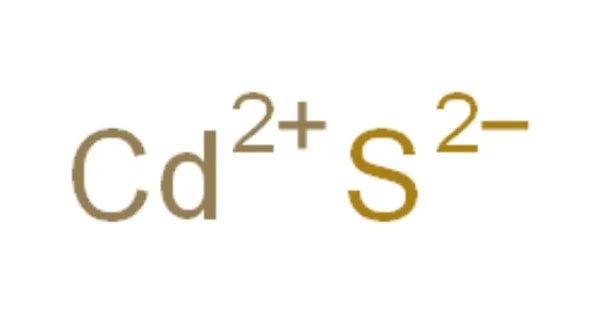Cadmium sulfide, abbreviated CdS, is an inorganic compound. Cadmium sulfide is a solid that is yellow in color. It can be found in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but it is more common as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. CdS is a yellow-brown, poisonous salt that is primarily used in electronic components, photoelectric cells, and medicine. It is the primary source of cadmium for all commercial applications because it is a simple compound to isolate and purify.
Cadmium sulfide is a pigment used in paints, plastics, textiles, ceramics, and glass, as well as solar cells, smoke and radiation detectors, light-emitting diodes, and photomultipliers. It is used as a colorant for paints and rubber; cadmium acetate is used in the production of craftware.
Properties
Cadmium Sulfide is an odorless, lemon yellow to orange crystal or yellow to brown powder. It is used in photoconductors, dandruff shampoos, pigments, electronic components, and solar cells.
- Chemical formula: CdS
- Molar mass: 144.47 g·mol−1
- Appearance: Yellow-orange to brown solid.
- Density: 4.826 g/cm3, solid.
- Melting point: 1,750 °C (3,180 °F; 2,020 K) 10 MPa
- Boiling point: 980 °C (1,800 °F; 1,250 K) (sublimation)
- Solubility in water: insoluble
- Solubility: soluble in acid very slightly soluble in ammonium hydroxide
- Crystal structure: Hexagonal, Cubic

Production
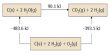
Cadmium sulfide can be made by precipitating soluble cadmium(II) salts with sulfide ion. This reaction has been used for gravimetric and qualitative inorganic analysis. The preparative route and subsequent treatment of the product have an impact on the polymorphic form that is produced (i.e., cubic vs hexagonal). It has been claimed that chemical precipitation methods yield cubic zincblende.
Pigment production typically entails precipitation of CdS, washing of the solid precipitate to remove soluble cadmium salts, calcination (roasting) to convert it to the hexagonal form, and milling to produce a powder. When cadmium sulfide selenides are required, CdSe is co-precipitated with CdS and the cadmium sulfoselenide is formed during the calcination step.
Cadmium sulfide is sometimes associated with sulfate reducing bacteria.
Structure
Cadmium sulfide, like zinc sulfide, exists in two crystal forms. The hexagonal wurtzite structure (found in the mineral Greenockite) and the cubic zinc blende structure are more stable (found in the mineral Hawleyite). Cadmium and sulfur atoms are four coordinate in both of these forms. A high pressure form with the NaCl rock salt structure is also available.
Cadmium sulfide is a direct band gap semiconductor (gap 2.42 eV). The proximity of its band gap to visible light wavelengths gives it a coloured appearance.
Applications
- Pigment
CdS is used as a pigment in plastics because of its high opacity, thermal stability, light and weather resistance, and chemical resistance. CdS is also known as cadmium yellow as a pigment (CI pigment yellow 37). As of 1982, approximately 2000 tons of cadmium were produced annually, accounting for approximately 25% of the cadmium processed commercially.
- Historical use in art
Cadmium sulfide became widely available commercially in the 1840s, prompting artists such as Van Gogh, Monet (in his London series and other works), and Matisse to use it (Bathers by a River 1916–1919). The presence of cadmium in paints has been used to detect forgeries in paintings allegedly created prior to the nineteenth century.
- CdS-CdSe solutions
With each other, CdS and CdSe form solid solutions. Increasing the amount of cadmium selenide results in red pigments, such as CI pigment orange 20 and CI pigment red 108. These solid solutions are components of photoresistors (light-dependent resistors) that are sensitive to visible and near-infrared light.
Safety
Exposure to this substance irritates the eyes, skin, and respiratory tract, damages the lungs, resulting in shortness of breath, chest pain, and pulmonary edema, and can also damage the kidneys, causing proteinuria and decreased renal function.
Cadmium sulfide is toxic, especially when inhaled as dust, and cadmium compounds in general are carcinogenic. When CdS is used as a color in tattoos, biocompatibility issues have been reported. Because of its low solubility, CdS has an LD50 of approximately 7,080 mg/kg in rats, which is higher than that of other cadmium compounds.