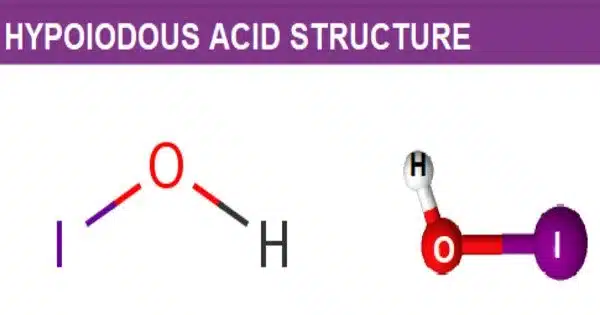
Hypoiodous acid is an inorganic compound with the chemical formula HIO. It is a chemical compound made up of hydrogen (H), iodine (I), and oxygen (O). It is formed when an aqueous solution of iodine is treated with mercuric or silver salts. Iodine and water combine to form a weak acid. Its chemical formula is HIO, and it is also known as hydroiodic acid. It decomposes quickly through disproportionation:
5 HIO → HIO3 + 2 I2 + 2 H2O
Hypoiodites of alkali and alkaline earth metals can be made in cold dilute solutions if iodine is added to their respective hydroxides. Hypoiodous acid is a weak acid with a pKa of about 11. The conjugate base is hypoiodite (IO−). Salts of this anion can be prepared by treating I2 with alkali hydroxides. They rapidly disproportionate to form iodides and iodates.
Properties
- Chemical formula: HIO
- Molar mass: 143.89 g/mol
- Acidity (pKa): 10.5 (in water, estimate)
- Conjugate base: Hypoiodite
Reaction
Hypoiodous acid is structurally and chemically similar to other halogen oxoacids such as hypochlorous acid (HOCl) and hypobromous acid (HOBr). It is an oxidizing agent with the ability to react with reducing agents. It is essential in certain chemical reactions and can help with disinfection processes. It is worth noting that hypoiodous acid has not been studied as thoroughly as some other halogen oxoacids, so its properties and applications may not be as well documented.
Hypoiodous acid is also known to be involved in some biological systems, most notably in the immune response of certain marine organisms and in the production of iodine-containing hormones by the thyroid gland.
Safety
Keep in mind that hypoiodous acid is a rare compound with limited properties and applications when compared to more well-known chemicals. If you’re working with or studying this compound, it’s critical to use proper safety precautions and rely on reliable chemical references for accurate information.