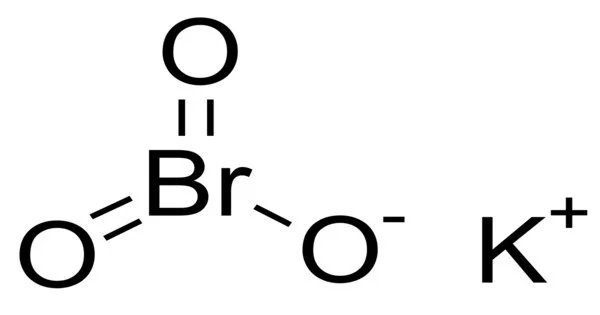
Potassium bromate is an inorganic compound with the formula KBrO3. It is a potassium bromate that comes in the form of white crystals or powder. It has high oxidizing power. It is a white solid that is soluble in water. It is used as a flour improver and a bleaching agent.
When bromine is passed through a hot potassium hydroxide solution, potassium bromate is formed. This produces unstable potassium hypobromite, which quickly disproportionates into bromide and bromated.
Properties
Potassium bromate appears as a white crystalline solid. It is a potassium salt and a bromate salt. It has a role as a flour treatment agent.
- Chemical formula: KBrO3
- Molar mass: 167.00 g/mol
- Appearance: white crystalline powder
- Density: 3.27 g/cm3
- Melting point: 350 °C (662 °F; 623 K)
- Boiling point: 370 °C (698 °F; 643 K) (decomposes)
- Solubility in water: 3.1 g/100 mL (0 °C); 49.7 g/100 mL (100 °C)
- Solubility: Insoluble in acetone
- Crystal structure: hexagonal

Preparation
When bromine is passed through a hot solution of potassium hydroxide, potassium bromate is formed. This first produces unstable potassium hypobromite, which rapidly degrades into bromide and bromate:
3BrO−(aq) → 2Br−(aq) + BrO−3(aq)
Bromate is produced by electrolysis of potassium bromide solutions. Both processes are similar to those used in the manufacture of chlorates. Due to its much lower solubility, potassium bromate is easily separated from potassium bromide in both methods; when a solution containing potassium bromate and bromide is cooled to 0°C, nearly all bromate precipitates while nearly all bromide remains in solution.
Applications
- Potassium Bromate is used as an oxidizer used to strengthen the dough and enhance its elasticity.
- It helps to bake uniform and whitened bread
- It is used as a flour improver
- It is used in the production of malt barley
- It is a source of bromine
- It is used in toothpaste as an antiseptic and astringent
Uses in baking
In the United States, potassium bromate is commonly used as a flour improver (E number E924). It strengthens the dough and allows for faster rising. It is an oxidizing agent that will be completely reacted to a form with a lower oxidation state in baking the bread under the right conditions. However, if too much is added, or if the bread is not baked long enough or at a high enough temperature, a residual amount may remain, which could be harmful if consumed.
Potassium bromate may also be used in the production of malt barley, for which the United States Food and Drug Administration (FDA) has established certain safety conditions, including labeling requirements for the finished malt barley product. It is a very powerful oxidizer (E° = 1.5 volts, comparable to potassium permanganate).
Health hazards
If ingested, KBrO3 is carcinogenic (causes DNA damage), neurotoxic, and nephrotoxic. The compound can cause hyperuricemia and thyroid follicular cell tumors. Because of the negative effects, many countries have banned its use as a food additive. Inhalation and skin and eye contact should be avoided because they can cause severe irritation and damage. When it comes into contact with organic material, it can cause a fire.