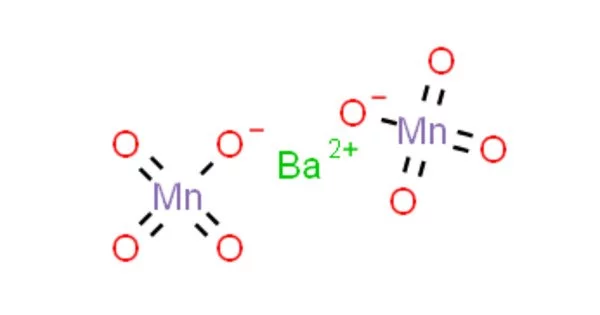
The chemical compound barium permanganate has the formula Ba(MnO4)2. It has the appearance of a purplish-colored crystalline solid. It is noncombustible, but it speeds up the combustion of combustible materials. It crystallizes into violet to brown crystals that are only slightly soluble in water. It is possible that finely divided combustible materials are explosive.
When it comes into contact with combustible liquids, it may spontaneously ignite. Sulfuric acid contact can cause fires or explosions. It is used as a disinfectant and in the production of other permanganates.
Properties
Barium Permanganate is a purplish-colored crystalline solid that is used as a disinfectant and in the production of other permanganates. Barium is an alkaline earth metal that is metallic. Due to its reactivity with air, it never occurs in its pure form in nature, but instead combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds, which can be found as minerals. Manganese is an element that occurs naturally. It does not occur naturally in its pure form, but it can be found in a variety of rocks in combination with other elements such as oxygen, sulfur, or chlorine.
- Chemical formula: Ba(MnO4)2
- Molar mass: 375.198 g/mol
- Appearance: dark violet to brown crystals
- Odor: odorless
- Density: 3.77 g/cm3
- Melting point: 200 °C (392 °F; 473 K) (decomposes)
- Solubility in water: 62.5 g/100 mL (29 °C)
- Solubility: decomposes in alcohol
- Crystal structure: rhombic

Preparation
Barium permanganate may be produced by disproportionation of barium manganate in a mildly acidic solution, including solutions carbon dioxide or sulfuric acid:
3 BaMnO4 + 2 CO2 → Ba(MnO4)2 + 2 BaCO3 + MnO2
3 BaMnO4 + 2 H2SO4 → Ba(MnO4)2 + 2 BaSO4 + MnO2 + 2 H2O
It is also possible to make it by oxidizing barium manganate with strong oxidants. Preparations based on aqueous reactions of barium manganate are extremely slow due to the manganate’s low solubility.
Another method for producing barium permanganate is through the reaction of silver permanganate and barium chloride. Highly pure samples can be obtained through a similar reaction between potassium permanganate and aluminum sulfate, which results in aluminium permanganate, which is then reacted with a stoichiometric amount of barium hydroxide.
Reactions
Barium permanganate is a strong oxidizer. It is thermally stable up to 180 °C, above which it decomposes in two stages between 180–350 and 500–700 °C.
2 Ba(MnO4)2 → 2 BaMnO3 + 2 MnO2 + 3 O2
4 BaMnO3 → 4 BaO + 2 Mn2O3 + O2
The decomposition has been shown to proceed at slow rates above 160 °C, and that irradiation with UV or X-rays lowers this temperature. Crystal defects and impurities play a role in the mechanism.
Permanganic acid can be prepared by the reaction of dilute sulfuric acid with a solution barium permanganate, the insoluble barium sulfate byproduct being removed by filtering:
Ba(MnO)2 + H2SO4 → 2 HMnO4 + BaSO4
The sulfuric acid used must be dilute; reactions of permanganates with concentrated sulfuric acid produce manganese heptoxide as anhydride.
It is a powerful oxidizer. When it comes into contact with combustible or finely divided materials/metals, it may become explosive. Keep sulfuric acid away from children; it is potentially explosive. Explosions can occur when sulfuric acid-treated permanganates come into contact with benzene, carbon disulfide, diethyl ether, ethyl alcohol, petroleum, or organic matter. Acetic acid or acetic anhydride can cause an explosion. Because violent reactions can occur, it is incompatible with acetic acid, acetic anhydride, and organic or combustible materials (such as wood, paper, oil, and fuels). This material, when mixed with combustible materials, can be ignited by friction or acids; it may be spontaneously combustible.