Measurement of Lowering of Vapour Pressure by Ostwald and Walker Method
A number of other devices have been developed for measurement of the difference of vapor pressure. All these are known as static methods to differentiate them from the dynamic method due to Ostwald and Walker which is described below. In this method, the relative lowering of vapor pressure can be determined directly. The measurement of the individual vapor pressures of a solution and solvent is thus eliminated.
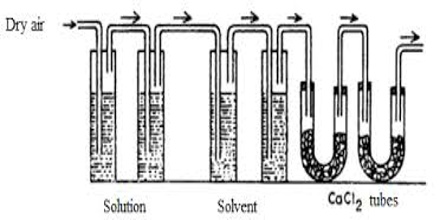
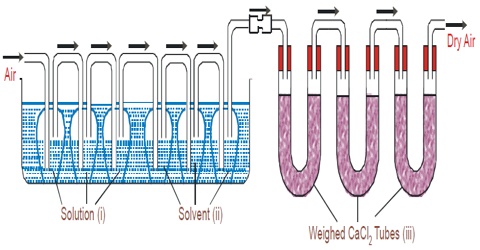
Figure: Transpiration method of Ostwald and Walker
In this method, a stream of dry air is bubbled successively through (i) the solution (ii) the pure solvent and (iii) a reagent which can absorb the vapor of the solvent. As the solvent is usually water the reagent is generally anhydrous Calcium Chloride.
Apparatus: The apparatus used is shown in the figure. It consists of two sets of bulbs. The first set of three bulbs is filled with the solution to half of their competence and second set of another three bulbs is filled with the untainted solvent. Each set is separately weighed accurately. Both sets are linked to each other and then with the accurately weighed set of guard tubes filled with anhydrous calcium chloride. The bulbs of the solution and pure solvent are reserved in a thermostat maintained at a stable temperature.
In this method, the solution and the solvent are taken in a series of gas washing bottles (Figure) and a slow stream of dry air or nitrogen is bubbled through the solution and the solvent. The first three bulbs surround a weighed amount of the solution under assessment and the next three bulbs surround a weighed amount of the pure solvent. A weighed amount of anhydrous calcium chloride is taken in the set of U-tubes at the end. The stream of gas coming out through D is saturated with the solvent vapor which is absorbed in a series of absorption tubes containing a suitable absorbing agent. The dry gas after passage through the solution becomes saturated with the solvent vapor from the pure solvent because the vapor pressure of the solvent is higher than that of the solution. The gas on passage through the solvent carries with it more vapor from it. After a sufficient flow of the gas, the absorption tubes and the two series of washing bottles are separately weighted. The increase in the mass of the absorption tube is proportional to the vapor pressure of the solvent and the loss in mass of the bottles containing solution is proportional to the vapor pressure of the solution, which loss in mass of the bottles, B, is proportioned to the lowering of vapor pressure. Thus,
(p0 – p) / P0 = [loss in mass of B / loss in mass of C]
Since the concentration of the solution is known the molecular mass of the solute is easily calculated. If temperature control is good and this experiment is carried out carefully, the method is capable of giving results of high accuracy. All the bulbs must be reserved at a similar temperature and air must be bubbled slowly to ensure that it gets saturated with the vapors in each bulb. The method is simple and inexpensive. If water is used as the solvent, the absorption tube may be filled with fused anhydrous calcium chloride. The last tube should be guarded against moisture absorption from the atmosphere. If organic solvents are used, as often is the case, the absorption tubes are dispensed with and the results can be obtained by weighing the tubes, A and B. The current of gas should be passed slowly otherwise fine stream of liquid drops may escape with the gas. The total volume of the gas passed should be large so that appreciable loss in mass in the two bulbs may occur.