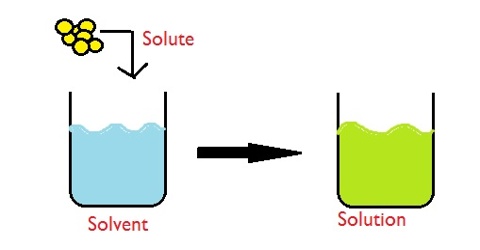
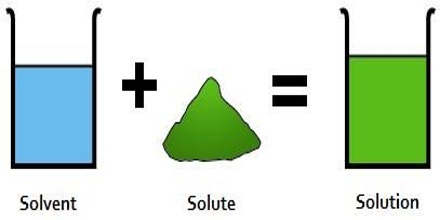
A solution is a system one comes across every day; it is so well known that it needs no explanation. If a little sugar is added to water, the sugar slowly disappears and sugar particles cannot be seen even under the most powerful microscope. It is said that sugar has dissolved in water to form a solution. A solution is usually defined as “a homogeneous mixture of two or more substances whose compositions can be varied over certain ranges”. This definition normally serves the purpose but a careful scrutiny reveals some complication.
If the molecules in a solution are picked up mentally one by one what could be seen? Once or twice a molecule of water would be picked up and once or twice a molecule of sugar could be picked up. Thus in a solution the molecules of water and sugar are lying side by side and state a molecule of water is different from a molecule of sugar that cannot be any homogeneity. Thus, judged on the molecular level, a solution is heterogeneous. But if a solution is considered on a macroscopic scale, then it is homogeneous since even in a fraction of a drop, millions of water and sugar molecules are present in a fixed proportion, thus giving homogeneity to the solution. The scale of our observation is thus important. Again, if a careful measurement of the number of solute molecules are made in a solution it will be found that the concentrations of the solute molecules are not the same in the solution bulk and on the solution surface. For practical purposes we still stick to the definition given above but we must bear in mind the incompleteness of the definition.
In a solution containing two constituents which has been described as above the constituent which is present in a larger amount is called the solvent and the one which is present in lesser amount is called the solute. The physical properties of solutions depend on the relative proportions of the components of which the solution is composed. If the components interact with each other then it is not a solution. It becomes a reaction.
Types of Solutions
Solutions may be divided into the following types:
(i) Gas in gas,
(ii) Gas in liquid,
(iii) Gas in solid,
(iv) Liquid in liquid,
(v) Liquid in solid,
(vi) Solid in solid,
(vii) Solid in liquid.