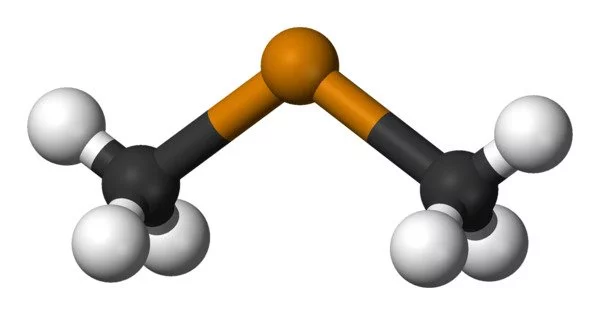
Dimethyl telluride, abbreviated DMTe, is an organotelluride chemical with the formula (CH3)2Te. It belongs to the alkyl telluride family, which is made up of tellurium atoms bound to alkyl groups. This was the first material utilised in metalorganic vapour phase epitaxy to generate epitaxial cadmium telluride and mercury cadmium telluride. The chemical is well recognized for its powerful and unpleasant odour, which has been characterised as smelling like garlic or decaying vegetables.
Dimethyl telluride was found in 1939 as a byproduct of microbial metabolism. Some fungus and bacteria (Penicillium brevicaule, P. chrysogenum, and P. notatum, as well as the bacterium Pseudomonas fluorescens) generate it.
Properties
- Appearance: It is a colorless gas at room temperature and standard pressure. It has a distinct garlic-like or “tellurium-like” odor, which can be detected even in low concentrations.
- Melting and boiling points: The melting point is around -38.5°C (-37.3°F), and its boiling point is approximately 92°C (198°F). These values can vary slightly depending on the purity of the compound.
- Density: The density of this gas is about 4.13 g/L at room temperature and standard pressure.
- Solubility: It is sparingly soluble in water, and its solubility decreases with increasing temperature. It is more soluble in organic solvents.
- Chemical reactivity: It is chemically reactive due to the presence of the tellurium atom. It can undergo various reactions, such as oxidation and substitution reactions, typical of organometallic compounds.
Production
Synthetically, dimethyl telluride can be made by reacting elemental tellurium with methylating chemicals such as dimethyl sulphate or dimethylzinc. It can also be produced as a byproduct of some industrial and biological processes.
Microbial activity is a substantial source of dimethyl telluride in the environment, particularly in the anaerobic environment of sediments and soils. Certain bacteria are capable of converting inorganic tellurium compounds into dimethyl telluride, which is then discharged into the environment.
Application
DMT has been explored for its possible applications in the detection and monitoring of tellurium compounds in the environment due to its pungent odour. It’s also employed in the semiconductor sector, specifically in the manufacture of metal telluride thin films for solar cells, infrared detectors, and thermoelectric devices.
Toxicity
Dimethyl telluride is extremely hazardous to humans and can be fatal if inhaled, consumed, or absorbed through the skin. Even at low quantities, inhalation can have serious health consequences, including respiratory, neurological, and gastrointestinal disorders.
When tellurium or one of its derivatives is consumed, the body produces it. It is recognised by the garlic-scented breath it gives persons who are exposed, which is comparable to the impact of DMSO.