Osmotic Pressure
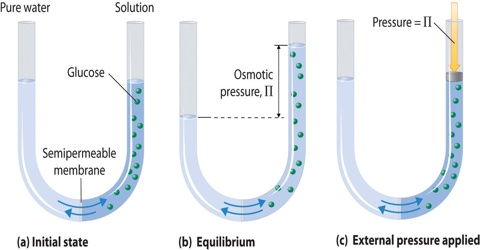
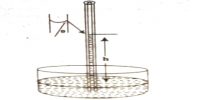
If a concentrated solution and a dilute solution of the same solute are placed together in the same container the solute particles move from the concentrated solution into the dilute solution. Due to difference in concentration the solvent molecules well go on migrating through the semi-permeable membrane unless it is presented by external forces to do so. This phenomenon of selective migration of the solvent, molecules through the separating semi-permeable membrane to the solution is called osmosis.
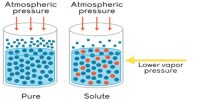
The osmotic pressure may, therefore, be defined as the external pressure that must be applied on the solution side to fast prevent the inflow of the solvent through the semi permeable membrane which separates the solvent and solution. If a membrane is present, water will flow to the area with the highest concentration of solute. Osmotic pressure is the pressure created by water moving across a membrane due to osmosis. The more water moving across the membrane, the higher the osmotic pressure. In this case the pressure, P shall be equal to the osmotic pressure.
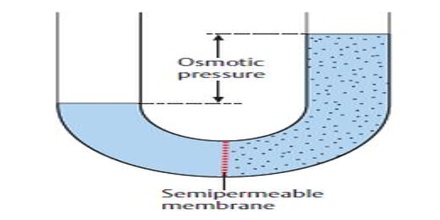
Fig: Osmotic pressure
If the semi -permeable membrane separates two solutions of different concentrations then also the solvent from the lower concentration side will flow to the higher concentration side. Thus concentration difference is the cause of osmotic pressure. Osmotic pressure is a colligative property.
It must be clearly understood that during migration of solvent molecules take place in both directions. But in osmosis the solvent molecules migrate in one direction only.