Scientists have created a new sodium metal anode for rechargeable batteries that resists the formation of dendrites, which is a common issue with standard sodium metal anodes and can lead to shorting and fires.
Researchers at the University of Texas at Austin have developed a new sodium-based battery material that is highly stable, capable of recharging as quickly as a traditional lithium-ion battery, and capable of delivering more energy than current battery technologies.
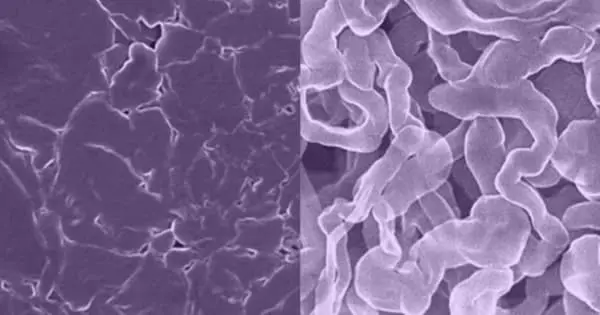
Scientists and engineers have been working on sodium batteries for about a decade, replacing both lithium and cobalt in current lithium-ion batteries with less expensive, more environmentally friendly sodium. Unfortunately, in older sodium batteries, a component is known as the anode tended to grow needle-like filaments known as dendrites, which can cause the battery to electrically short and even catch fire or explode.
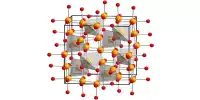
According to our calculations, this composite binds sodium slightly more strongly than sodium binds itself, which is the ideal case for having the sodium atoms come down and evenly spread out on the surface and preventing these instabilities from forming.
Graeme Henkelman
The new material solves the dendrite problem and recharges as quickly as a lithium-ion battery in one of two recent sodium battery advances from UT Austin. The team’s findings were published in the journal Advanced Materials.
“We’re essentially solving two problems at once,” said David Mitlin, the new material’s designer and a professor in the Cockrell School of Engineering’s Walker Department of Mechanical Engineering and Applied Research Laboratory. “Typically, the faster you charge, the more dendrites you grow, so if you suppress dendrite growth, you can charge and discharge faster because it’s suddenly safe.”
Graeme Henkelman, a professor in the Department of Chemistry and the Oden Institute for Computational Engineering and Sciences, used a computer model to explain why the material has the unique properties it does from a theoretical standpoint.

“This material is also exciting because the sodium metal anode has the highest theoretical energy density of any sodium anode,” Henkelman said.
The demand for stationary energy storage systems for homes and for smoothing the ebb and flow of wind and solar energy on electric grids is increasing. Simultaneously, lithium mining has been chastised for its environmental impacts, which include excessive groundwater use, soil and water pollution, and carbon emissions. Lithium-ion batteries typically contain cobalt, which is expensive and is primarily mined in the Democratic Republic of the Congo, where it has serious consequences for human health and the environment. Sodium mining, on the other hand, is less expensive and more environmentally friendly.
Mitlin believes that this new innovation, along with others from UT Austin, such as a new solid electrolyte that improves energy storage, will mean that sodium batteries will soon be able to meet the growing demand for stationary energy storage.
Ions (such as lithium or sodium) move from one component called the cathode to another called the anode when a rechargeable battery is charged. Ions move from the anode to the cathode when the battery is used to generate electricity.
The sodium antimony telluride intermetallic — Na metal composite (NST-Na) anode material is created by rolling a thin sheet of sodium metal onto an antimony telluride powder, folding it over on itself, and repeating many times.
“Think about making a layered pastry, like spanakopita,” Mitlin suggested.
This process produces a very uniform distribution of sodium atoms, making it less likely than existing sodium metal anodes to form dendrites or surface corrosion. This increases the battery’s stability and allows for faster charging, comparable to the charge rate of a lithium-ion battery. It also has a greater energy capacity than current sodium-ion batteries.
According to Henkelman, if the sodium atoms that carry a charge in a sodium battery bind more strongly to each other than to the anode, instabilities, or clumps of sodium, form, which attract more sodium atoms and eventually lead to dendrites. He used a computer simulation to show what happens when individual sodium atoms interact with the new NST-Na composite material.
“According to our calculations, this composite binds sodium slightly more strongly than sodium binds itself, which is the ideal case for having the sodium atoms come down and evenly spread out on the surface and preventing these instabilities from forming,” Henkelman said.