Ammonia production is responsible for about one percent of greenhouse gas emissions, although it cannot reduce. It does not sound like much, but we think a lot about the emissions of air travel, which is not much bigger than that. It is unlikely for about 8 billion people to live in this world without ammonia-based fertilizers, but we need to find a better way. Australian scientists think they are very close. Ammonia (NH3) production currently relies on the Haber-Bosch process – which won the Nobel Prize in 1918 – but the need for replacement is dire. The process uses a lot of energy and relies on hydrogen, usually produced from methane, a few of which leak. “Green ammonia” can made using renewable energy and electrolysis of water, but it is currently very expensive.
Dr Emma Lovell of the University of New South Wales told IFLScience, “The problem with using electricity to convert nitrogen directly into ammonia is that nitrogen is so stable that it is very difficult to dissolve in water.” “So we took a step back and thought about how nature does it.”
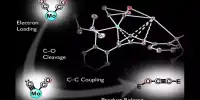
Lightning converts some atmospheres into NOx molecules, which then converted into other compounds, allowing this essential element to form part of every living thing. Lovell and colleagues began to think of ways to replace it. Perhaps, others had the same idea, but Lovell thinks that the previous work “does not overlap too much in plasma physics and electronics. We are just lucky that one person working in each got a coffee together and from there things move on.” In Energy and Environmental Science Lovell and other team members describe a plasma bubble column reactor that first converts atmospheric nitrogen into NOx like lightning, and then electrolyzes water to make hydrogen that displaces the oxygen.
Starting with a highly inefficient process, Love told IFLScience that the energy consumption of ammonia per gram has reduced by 100 times. He thinks two or three more improvements are possible, which could eventually throw Haber-Bosch his perch. In addition to the environmental costs of producing ammonia, the efficiency of existing methods requires giant manufacturing plants, which means the product shipped worldwide. Last year, 3,000 tonnes of ammonium nitrate fertilizer stored in the port of Beirut exploded, killing 300,000 homeless people and killing at least 204 people, reminding the world of those dangers.
In addition to not requiring fossil fuels, Lovell’s method can work in a variety of sizes. Lovell said in a statement, “The technology can be used to produce ammonia directly on site and on demand … which means we ignore storage and transportation requirements.” The team is working on a design that can operate on the farm without air, water and a few solar panels.
Either way the NOx gases are locally toxic or not a greenhouse pollutant, but Love told IFLScience that he suspects the leaking system he is building will allow any leaks before it converted to ammonia.
Solving one of the world’s biggest environmental challenges may be enough for most people, but Lovell and his colleagues think they can help with a larger one. Hydrogen carries a lot of hope as a way to store and transport energy from places rich in sunlight and air.
“Hydrogen is very light, so you need a lot of space to store it, otherwise it will have to be compressed or liquefied, but liquid ammonia actually stores more hydrogen than liquid hydrogen,” noted senior author Professor Rose Amal. Recent advances in the easy separation of ammonia nitrogen and hydrogen increase the likelihood of solving many of the hydrogen problems of ammonia transport.