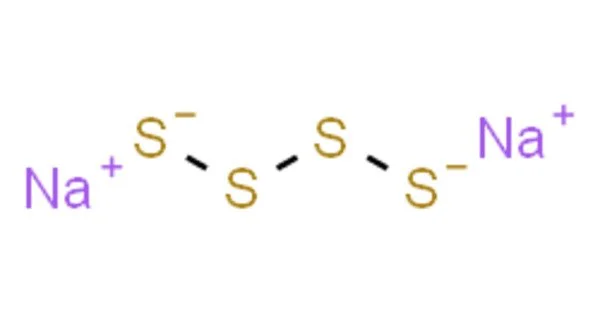Sodium tetrasulfide is an inorganic compound with the formula Na2S4. It is a compound formed by the combination of sodium (Na) and sulfur (S). It is a yellow-orange solid that dissolves via hydrolysis in water. It is a precursor to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery.
Sodium sulfide is typically found as a colorless or yellowish solid. It can also exist as a solution in water. It is known for its unpleasant odor, often described as rotten eggs. This odor is due to the presence of hydrogen sulfide gas. Sulfides, in general, are compounds containing the sulfide ion (S2-). Sodium sulfide itself is a well-known compound and is commonly used in various industries.
Properties
- Chemical formula: Na2S4
- Molar mass: 174.24g/mol
- Appearance: Dark red, slightly viscous liquid or yellow crystalline powder
- Density: 1.268 g/cm3 at 15.5 °C
- Melting point: 275 °C (527 °F; 548 K)
- Solubility in water: Soluble in water
Synthesis and structure
Sodium sulfide is soluble in water. The solubility may vary depending on temperature. It It is a reactive compound, and its reactivity can increase in the presence of acids. It can be corrosive to metals, especially in the presence of moisture. It is produced through the reaction between elemental sulfur and sodium hydrosulfide in alcoholic solution:
2NaSH + 4 S → Na2S4 + H2S
The polysulfide anions adopt zig-zag chains of sulfur atoms. The S-S distances are about 2.05 Å and the S-S-S-S dihedral angles are around 90°.
Application
Sodium tetrasulfide can be used as a reducing agent in organic synthesis. It is known to reduce certain functional groups, such as nitro groups, to their corresponding amines. It can serve as a source of sulfur in chemical reactions, contributing to the synthesis of sulfur-containing compounds.
Sodium tetrasulfide is used in the preparation of metal sulfides. By reacting with metal ions, it can lead to the formation of various metal sulfides, which find applications in areas like catalysis and semiconductor materials.