Observation of Electrical Conductivity of Metal:
Required Accessories: One electric cell (battery), one electrical bulb, 2 electrical wire, steel spoon, piece of aluminum. Rubber, wood and plastic spoon.
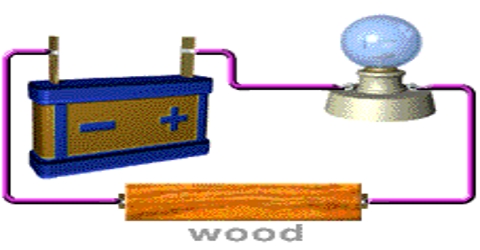
Procedure: Take the electric cell and see that there is a positive (+) sign at one end and negative (-) sign at other end. Connect one coma wire at one end of the cell and another copper wire at the other end of the cell (as in the fig). Now you take the electrical bulb. You will see two raised metallic points or thick wire like points at one end of the bulb which end is suppose to go into the socket. Now connect the open end of one wire with one of the connecting points of the bulb and connect the other wire with another connecting point.
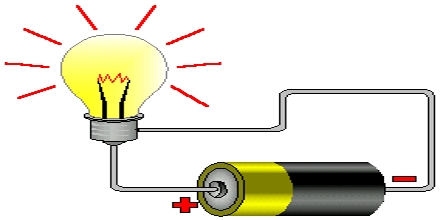
Analysis: What do you see? The bulb is illuminating. As copper is an electric conductor, it transmits electricity taking from the cell and send it to the bulb. For this reason the bulb lights up. If the copper wire was not an electric conductor, it could not conduct electricity, and the bulb would not illuminate.
Now disconnect the copper wire and give connection between them with iron or aluminum wire. See what happens? Next connect the two wires by piece of wood, plastic, rubber etc.
Has the electric bulb illuminated now? No, it has not. Since rubber, plastic and piece of wood- all these are electric non-conductors, they cannot conduct electric charges from the cell. That’s why the bulb has not illuminated.