Artificial Intelligence use to unlock the nature of chemical bonding
Researchers have developed a Bayesian chemisorption learning model, or Bayeschem in short, to unlock the essence of chemical bonding on a catalytic surfaces by using artificial intelligence. A recent methodology in machine learning provides valuable insights into catalysis, a basic mechanism that allows the reduction or production of critical materials such as fabrics in toxic exhaust gas emissions.
Hongliang Xin, associate professor of Virginia Tech’s chemical engineering, developed a Bayesian model for chemisorption, or short Bayeschem, to unlock the nature of chemical bonds on catalytic surfaces, in a paper published in Nature Communications.
Using machine learning in chemistry enhances this theory’s level, enabling higher accuracy of prediction and interpretation. It measures the strength of the chemical bonds and makes it possible to tweak the composition or structure for materials with better adsorption properties.
The sum of the contact of substrates with catalysts depends on the type and frequency of the chemical relation. Chemisorption happens on catalytic surfaces. Xin explained the catalytic theory of Sabatier that catalytic contact is intense enough to separate chemical bonds at a pleasantly low temperature, but this strength must still be stabilised to a medium degree such that intermediate compounds do not become intoxicating.
“It all comes down to how catalysts bind with molecules,” Xin said. “The contact should be strong enough to sever such chemical interactions at relatively low temperatures but not so strong for catalysts to be poisoned by intermediate reactions.
Understanding how catalysts interact with various intermediates and how their bond strengths are controlled to be within this “goldilocks zone” is important to design effective catalytic processes, said Xin. For this reason, the research offers a tool.
Bayeschem uses a Bayesian study algorithm, a special computer for testing data models. “Suppose you have a domain model based on well-established physical laws, and you want to use it to make predictions or learn something new about the world says Siwen Wang, the old Ph.D. student in chemistry. “The Bayesian approach is to learn the distribution of model parameters given our prior knowledge and the observed, often scarce, data while providing uncertainty quantification of model predictions.”
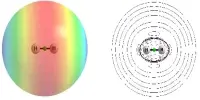
The Bayeschem d-band chemisorption theory is a theory explaining chemistry bonds in solid surfaces, which typically have a four-leaf pattern, using d-electrons. The model describes the overlapping and adsorption of valence orbits that have the spherical or stupidity structure by d-orbitals of catalyst atoms. Since Hammer and Nørskov established the standard method in heterogeneous catalysis in the 90s, Xin said the pattern was often failed due to the difficulty of electronic interactions, even though it was effective in explained relations between the different systems.
The Bayeschem d-band chemisorption theory is a theory explaining chemistry bonds in solid surfaces, which typically have a four-leaf pattern, using d-electrons. The model describes the overlapping and adsorption of valence orbits that have the spherical or stupidity structure by d-orbitals of catalyst atoms. Since its development by Hammer and Nørskov in the 1990s, it has been considered a basic model in heterogeneous catalysis, and while it has been effective in understanding the bonding patterns of many structures, Xin has argued that the model often fails due to the inherent difficulty of electronic interactions.
“The opportunity to come up with highly accurate and interpretable models that build on deep learning algorithms and the theory of chemisorption is highly rewarding for achieving the goals of artificial intelligence in catalysis,” Xin said.
Xin concluded that artificial catalysis intelligence has several objectives that involve reliable and computational models that enhance deep learning algorithms. The chance to do this when uncovering more secrets of chemisorption would be immensely rewarding.