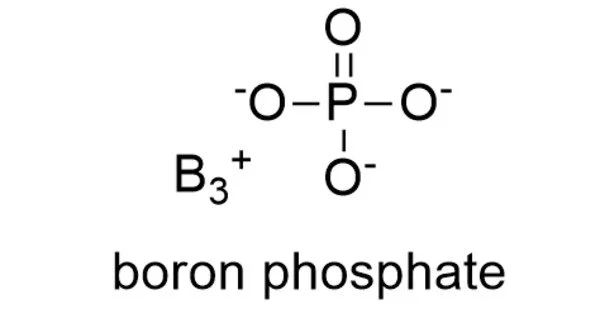
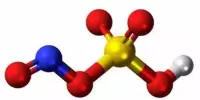
Boron phosphate has the chemical formula BPO4 and is an inorganic substance. The reaction of phosphoric acid with boric acid is the most basic method of making it. It can exist in a variety of forms or phases, with various qualities depending on the phase. It is a white infusible solid that evaporates at temperatures above 1450 °C.
Crystal Structure
Boron phosphate has a crystalline structure in most cases. Depending on the synthesis circumstances and the type of boron phosphate generated, the crystal lattice configuration can vary. It is a tough substance with great abrasion resistance. This makes it excellent for applications requiring hardness, such as ceramics and cutting tools.
Thermal Stability
Boron phosphate has a high thermal stability. It can sustain high temperatures without decomposing or going through phase changes. Because of its feature, it can be used in high-temperature applications such as refractory materials. Because it is an electrical insulator, it does not conduct electricity. This feature makes it helpful in situations requiring electrical insulation.
Synthesis
Boron phosphate is made by combining phosphoric acid and boric acid at temperatures ranging from 80 °C to 1200 °C. The comparatively cold treatment yields a white amorphous powder, which is transformed to a microcrystalline product after 2 hours of heating at around 1000 °C.
The main reaction of the process is:
H3BO3 + H3PO4 → BPO4 + 3 H2O
New ways of synthesizing the compound have also been reported, such as hydrothermal and microwave synthesis. Due to the particular industrial interest of boron phosphate, other methods are used as well:
- Phosphoric acid and triethyl borate
- Triethyl phosphate and boron trichloride
- Diammonium phosphate acid and borax heated to 1000 °C
- Boric acid and phosphorus pentoxide (hydrothermal)
Here are a few common phases of BPO4:
- α-Boron Phosphate: This is the most stable phase of boron phosphate at room temperature and standard pressure. It has a crystalline structure and is an electrical insulator.
- β-Boron Phosphate: This phase is metastable at room temperature but may transform into the α-phase at higher temperatures. It exhibits different properties from the α-phase.
- γ-Boron Phosphate: This phase can be obtained by heating α-Boron phosphate to high temperatures and then rapidly cooling it. It has a different crystal structure and properties.
Structure
If obtained at pressure, the ordinary structure is isomorphous with the β-cristobalite, while subjecting it to high pressure is obtained a compound isomorphic with α-quartz. The structure of AlPO4, berlinite, is isomorphous with α-quartz.
Applications
It is employed as a catalyst in organic synthesis for dehydration and other processes. It also acts as a source of phosphates for solid-state exchange processes to produce metal phosphates. It has numerous uses in materials science and chemistry. It is utilized in the creation of specialty materials with specific electrical and thermal properties, as well as the synthesis of certain types of glasses and ceramics. It can also be used as a catalyst in some chemical processes.
Safety Precautions
While boron phosphate is generally regarded harmless, suitable safety precautions must be taken when handling it. Boron compounds can be dangerous if inhaled or ingested, and skin contact should be avoided. Safety data sheets for specific boron phosphate products should be consulted for detailed safety information.