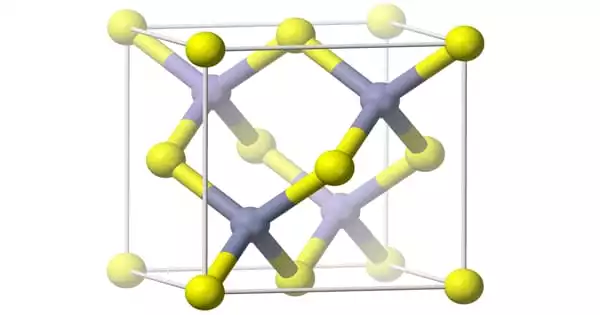
Aluminium arsenide (AlAs) is a semiconductor with a nearly identical lattice constant to gallium arsenide and a larger band gap than gallium arsenide. Aluminum arsenide (AlAs) can form a superlattice with gallium arsenide (GaAs), resulting in semiconductor characteristics. Because GaAs and AlAs have nearly identical lattice constants, the layers have negligible induced strain and can be produced practically arbitrarily thick. This enables high electron mobility, HEMT transistors, and other quantum well devices to have extraordinarily high performance.
Aluminum arsenide reacts quickly with acids, acid gases, and moisture. Arsenic gas and arsenic fumes will be produced during the decomposition of aluminum arsenide. It contains the ions aluminum and arsenide.
Properties
Aluminium arsenide is an orange solid. It is toxic because it has arsenic in it. It is a semiconductor. It reacts with acids to make arsine. It is made by reacting aluminium and arsenic. It has the following properties:
- Thermal expansion coefficient 5 µm/(°C*m)
- Debye temperature: 417 K
- Microhardness: 5.0 GPa (50 g load)
- Number of atoms in 1 cm3: (4.42-0.17x)·1022
- Bulk modulus (7.55+0.26x)·1011 dyn cm−2
- Hardness on the Mohs scale: ~ 5
- Insolubility in H2O
- Molecular Weight: 101.9
- Appearance: Gray powder or pieces
- Melting Point: 1740 °C
- Boiling Point: N/A
- Density: 3.76-3.8 g/cm3
Uses
Aluminum arsenide is a III-V compound semiconductor material that is useful in the production of optoelectronic devices such as light emitting diodes. It can be made using well-known processes like as liquid and vapor-phase epitaxy or melt-growth procedures. However, when exposed to wet air, aluminum arsenide crystals generated by these procedures are often unstable and produce arsine (AsH3).
Synthesis
Because of the practical challenges involved, little work on the preparation of aluminum arsenide has been documented. Because of the compound’s high melting point (about 1,700 °C) and the severe reactivity of aluminum at this temperature, preparation from the melt is difficult. A few employees have created tiny crystals from the melt, as well as polycrystalline ingots. The best of this material is p-type and has an impurity carrier density of the order of 1019/cm3.
Reactivity
Although aluminum arsenide is a stable product, it should be kept away from acids, acid vapors, and moisture. There will be no hazardous polymerization. Aluminum arsenide decomposition creates dangerous arsine gas and arsenic fumes.
Toxicity
Aluminum arsenide’s chemical, physical, and toxicological properties have not been adequately researched and reported. Aluminum compounds have a wide range of commercial applications and are widely used in industry. Many of these compounds are chemically active and so have hazardous toxic and reactive qualities.