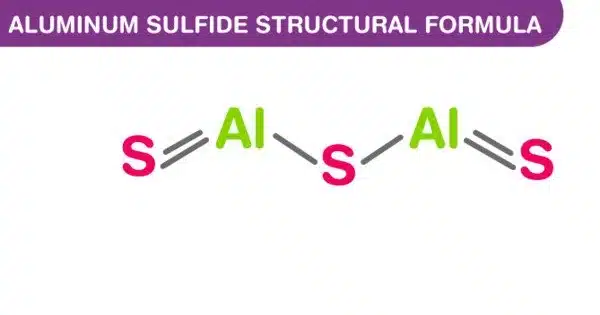
Aluminium sulfide is a chemical compound with the formula Al2S3. It is an aluminum and sulfur compound. This colorless species has an intriguing structural chemistry and exists in several forms. The material is moisture sensitive, hydrolyzing to hydrated aluminum oxides/hydroxides. It is classified as a binary ionic compound, which means it contains ions from two different elements. This can happen when the sulfide is exposed to air. The hydrolysis reaction produces gaseous hydrogen sulfide (H2S).
Aluminum sulfide can exist in a variety of crystal structures. The most common are hexagonal and rhombohedral. It reacts with acids to produce hydrogen sulfide gas and an aluminum salt.
Properties
- Chemical Formula: Al2S3
- Molecular Weight: approximately 150.16 g/mol.
- Appearance: It is a white or grayish-white solid in its pure form.
- State: It exists in the solid state at room temperature.
- Melting Point: around 1,100 to 1,130 degrees Celsius (2,012 to 2,066 degrees Fahrenheit).
- Density: approximately 2.02 g/cm³.
- Solubility: It is insoluble in water. It hydrolyzes in water to produce hydrogen sulfide gas and aluminum hydroxide.
Preparation
Aluminium sulfide is readily prepared by ignition of the elements –
2 Al + 3 S → Al2S3
This reaction is extremely exothermic and it is not necessary or desirable to heat the whole mass of the sulfur-aluminium mixture; (except possibly for very small amounts of reactants). The product will be created in a fused form; it reaches a temperature greater than 1100 °C and may melt its way through steel. The cooled product is very hard.
Use
Aluminum sulfide has limited commercial applications, but it may be used in certain chemical processes and research applications.
Hazards
Hydrogen sulfide gas produced during the reaction with water is toxic and poses health risks.