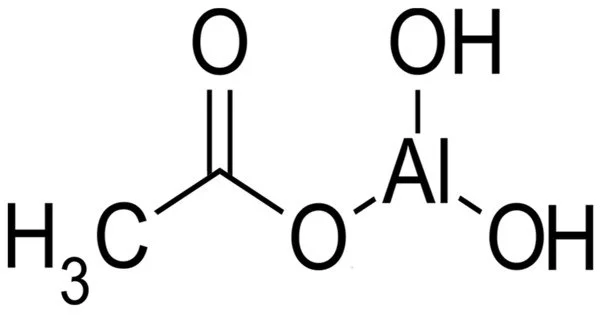
Aluminium monoacetate, is a salt of aluminium with acetic acid. It has the formula Al(OH)2(CH3COO), with aluminium in an oxidation state of +3, and appears under standard conditions as a white solid powder. It is also known as basic aluminum acetate or aluminum subacetate. It is a white crystalline solid that is soluble in water and has a slight acidic odor. It is formed by the reaction of aluminum hydroxide with acetic acid.
Aluminium monoacetate is used in various industries such as the cosmetic industry as an astringent and antiseptic agent, in the pharmaceutical industry as a topical astringent and antiseptic, and in the paper industry as a sizing agent. It is used in the production of antiperspirants, astringents, and other cosmetic products due to its ability to reduce sweat gland activity and tighten skin.
Properties
- Chemical formula: (HO)2AlCH3CO2 or C2H5AlO4
- Molar mass: 120.04 g/mol
- Appearance: White powder
- Solubility: It is soluble in water and forms a slightly acidic solution
- pH: The pH of a 1% solution of aluminum monoacetate is around 4
- Stability: It is stable under normal conditions but decomposes when heated
Preparation
Aluminium monoacetate is prepared by reacting aluminum hydroxide with acetic acid, and the resulting product is then purified and dried. The compound can also be obtained by reacting aluminum sulfate with sodium acetate. It is prepared from the reaction between Al(OH)3 and dilute aqueous acetic acid. It is also formed from the successive hydrolysis of aluminium triacetate.
Al(CH3COO)3 + H2O → Al(OH)(CH3COO)2 + CH3COOH
Al(OH)(CH3COO)2 + H2O → Al(OH)2(CH3COO) + CH3COOH
Uses
Aluminium monoacetate is an antiseptic and astringent dermatological agent. It is used as an antiseptic to prevent infection in minor wounds, cuts, and burns. It specifically treats itching, stinging, inflammation, and promotes healing of infected skin. When applied to the skin, it can also be used as a topical astringent to help shrink the body’s tissues by acting as a protective layer on irritated and inflamed skin.
While aluminum monoacetate is generally considered safe for use in various applications, excessive exposure to aluminum can be harmful to human health. Long-term exposure to high levels of aluminum has been associated with neurotoxicity and may contribute to the development of Alzheimer’s disease. Therefore, it should be handled with care and used only in the recommended amounts.
Toxicity
Aluminum monoacetate is considered safe for use in cosmetic products, but ingestion or inhalation of large amounts may cause irritation of the digestive or respiratory system.