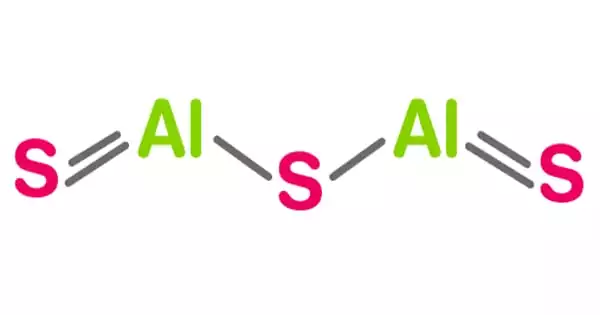
Aluminum sulfide is a chemical compound having the formula Al2S3. This colorless compound has an intriguing structural chemistry and exists in a variety of forms. The material is moisture sensitive and hydrolyzes to hydrated aluminum oxides/hydroxides. This can start when the sulfide is exposed to air. Gaseous hydrogen sulfide is produced during the hydrolysis reaction (H2S). It is a raw material in the production of the widely utilized hydrogen sulfide.
Aluminum sulfide, like other metal sulfides, is mildly soluble in water but very soluble in acid solutions. In the presence of sodium or potassium bases, it can react to generate sodium sulfide, an irritant that can cause skin and eye irritation.
Properties
Aluminum sulfide is a gray to black solid. Its density is 2.02 g mL-1. The melting point of this compound is 1100 °C and the boiling point is 1500 °C, at this temperature it sublimes. Aluminum sulfide decomposes in water and is insoluble in acetone.
- Chemical formula: Al2S3
- Molar mass: 150.158 g/mol
- Appearance: gray solid
- Density: 2.02 g/cm3
- Melting point: 1,100 °C (2,010 °F; 1,370 K)
- Boiling point: 1,500 °C (2,730 °F; 1,770 K) sublimes
- Solubility in water: decomposes
- Solubility: insoluble in acetone
Formula
Aluminum sulfide chemical formula is Al2S3 and the molar mass is 150.16 g mol-1. Sometimes this sulfide can be found in its hydrate form, with a variable number of water molecules hydrating it. The compound is formed by two cation Al3+ and three anions S2-.

Preparation
Aluminum sulfide is prepared by the reaction of the aluminum metal with sulfur powder. They are heated to ignition to form the sulfide though a highly exothermic reaction:
2 Al + 3 S → Al2S3
This reaction is extremely exothermic, and it is neither required nor desirable to heat the entire mass of the sulfur-aluminum mixture (except possibly for very small amounts of reactants). The product will be fused; it will reach temperatures more than 1100 °C and may melt through steel. The cooled product is extremely hard.
Uses
Aluminum sulfide is utilized in the production of hydrogen sulfide, a substance widely employed in the chemical industry. It is also utilized in the production of cathodes for lithium-sulfur solid-state batteries.
- Used in the preparation of hydrogen sulfide.
- Used to produce chemicals used in the tanning and papermaking industry.
- Used to produce organic compounds such as ethanethiol.
Safety Hazards
Aluminum sulfide is extremely poisonous and hazardous to one’s health. It is toxic if inhaled. It can cause serious nerve damage, and its accumulation in the body can also cause blood damage.