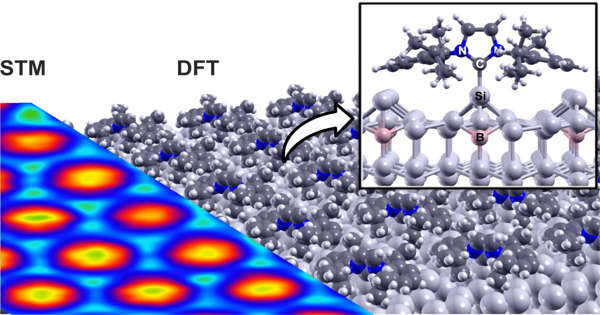
An interdisciplinary team of researchers has succeeded in depositing a highly ordered layer of nitrogen-containing organic molecules on silicon. This opens up new avenues for the development of more powerful semiconductor materials, which could be used in applications such as computers, photovoltaics, and sensor technology.
One vision that is currently motivating material scientists is the combination of organic molecules (and their diverse functionalities) with the technological possibilities provided by extremely sophisticated semiconductor electronics. The latter creates ever more efficient electronic components for a wide range of applications thanks to modern micro and nanotechnology methods. However, it is also approaching its physical limits: Classical technology approaches cannot produce ever smaller structures for functionalizing semiconductor materials such as silicon.
In the journal Nature Chemistry, scientists have now presented a new approach: They demonstrate that self-assembly can produce stable and well-ordered molecular single layers on silicon surfaces. They do this by using N-heterocyclic carbenes. These are small reactive organic ring molecules with a wide range of structures and properties that can be tailored by various “functional” groups.
Researchers succeeded in depositing nitrogen-containing organic molecules as a highly ordered layer on silicon. This opens up new perspectives for the development of more powerful semiconductor materials.
Researchers led by Prof. Dr. Mario Dähne (TU Berlin, Germany), Prof. Dr. Norbert Esser (TU Berlin and Leibniz Institute for Analytical Sciences, Germany), Prof. Dr. Frank Glorius (University of Münster, Germany), Dr. Conor Hogan (Institute of Structure of Matter, National Research Council of Italy, Rome, Italy) and Prof. Dr. Wolf Gero Schmidt (University of Paderborn, Germany) were involved in the study.
Technological miniaturization reaches its limits
“Rather than attempting to artificially produce smaller and smaller structures with increasing effort,” says chemist Frank Glorius, “it is obvious to learn from molecular structures and processes in nature and to merge their functionality with semiconductor technology.” “This would serve as a bridge between molecular function and electronic user interface for technical applications.” The ultra-small molecules with variable structure and functionality must be physically incorporated with semiconductor devices, and they must be reproducible, stable, and as simple as possible.
Harnessing the self-organization of molecules
This task can be very well performed by the self-organization of molecules on a surface as an interface to the device. Molecules with a defined structure can be adsorbed in large numbers on surfaces and arrange themselves into the desired structure determined by molecular properties. “This works quite well on metal surfaces, for example, but not at all satisfactorily for semiconductor materials so far,” physicist Norbert Esser explains. This is due to the fact that in order for molecules to arrange themselves, they must be mobile (diffuse) on the surface. However, molecules on semiconductor surfaces do not behave in this manner. Rather, they are so strongly bound to the surface that they stick wherever they hit the surface.
N-Heterocyclic carbenes as a solution
Being mobile while remaining stably bonded to the surface is the critical problem, as well as the key to potential applications. And it is precisely here that the researchers have a potential solution: Carbenes that are N-heterocyclic. Over the last decade, there has been a lot of interest in their use for surface functionalization. They have proven to be very effective surface ligands on metal surfaces such as gold, silver, and copper, often outperforming other molecules. Their interaction with semiconductor surfaces, on the other hand, has largely gone unexplored.
Formation of a regular molecular structure
Certain carbene properties are critical to the fact that it is now possible to produce molecular single layers on silicon surfaces for the first time: N-heterocyclic carbenes, like other molecules, form extremely strong covalent bonds with silicon, allowing them to be stably bound. However, the molecule’s side groups keep them “at a distance” from the surface. As a result, they can still move around on the surface. They travel only a few atomic distances, but this is enough to form an almost equally regular molecular structure on the surface of the regularly structured silicon crystal.
Interdisciplinary collaboration
In their interdisciplinary collaboration, the researchers clarified the principle of this novel chemical interaction using a complementary multi-method approach of organic chemical synthesis, scanning probe microscopy, photoelectron spectroscopy, and comprehensive material simulations. In several examples, they also demonstrated the formation of regular molecular structures. “This opens a new chapter for the functionalization of semiconductor materials, such as silicon in this case,” says first author and physicist Dr. Martin Franz.