Calcium hydroxide (also known as slaked lime) is an inorganic compound with the formula Ca(OH)2. When quicklime (calcium oxide) is mixed with water, it produces a colorless crystal or white powder. It is known by several different names, including hydrated lime, caustic lime, builders’ lime, slaked lime, cal, and pickling lime.
Calcium hydroxide is used in a variety of applications, including food preparation, and has the E number E526. A saturated solution of calcium hydroxide is known as limewater, also known as milk of lime.
Properties
Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. It is an inorganic compound that has a white, powdery appearance in its solid state. However, Ca(OH)2 has a colorless appearance in its crystalline form.
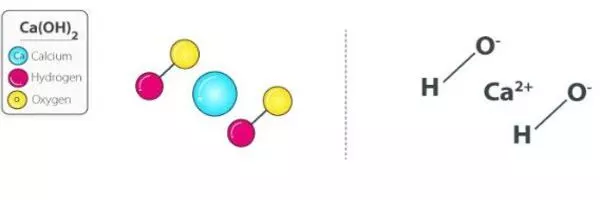
- Chemical formula: Ca(OH)2
- Molar mass: 74.093 g/mol
- Appearance: White powder
- Odor: Odorless
- Density: 2.211 g/cm3, solid
- Melting point: 580 °C (1,076 °F; 853 K) (loses water, decomposes)
- Solubility in water: 1.89 g/L (0 °C); 0.66 g/L (100 °C)
- Soluble: glycerol and acids
- Insoluble: in ethanol.
- Crystal structure: Hexagonal, hP3
Preparation
Calcium hydroxide adopts a polymeric structure, as do all metal hydroxides. The structure is identical to that of Mg(OH)2 (brucite structure); i.e., the cadmium iodide motif. Strong hydrogen bonds exist between the layers.
Calcium hydroxide is produced commercially by treating (slaking) lime with water:
CaO + H2O → Ca(OH)2
It can be made in the laboratory by combining aqueous solutions of calcium chloride and sodium hydroxide. Portlandite, a mineral form, is relatively rare but can be found in some volcanic, plutonic, and metamorphic rocks. It has also been observed in burning coal dumps.
Ca(OH)2 is highly soluble in glycerol and acids, but only marginally soluble in water. When dissolved in water to saturation, it produces a solution that acts as a moderate base (called limewater).
When limewater reacts with acids, salts form. Metals such as aluminum are also reacted with and dissolved by a saturated solution of calcium hydroxide in water. Calcium carbonate is formed when it reacts with carbon dioxide (CaCO3). Carbonatation is the common name for this reaction.
Uses
Calcium hydroxide is a common ingredient in lime mortar. One important application of calcium hydroxide is as a flocculant in water and sewage treatment. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. The low cost and low toxicity of calcium hydroxide enable this application. It is also used in fresh-water treatment to raise the pH of the water so that pipes do not corrode where the base water is acidic, because it is self-regulating and does not raise the pH too much.
- In the process of sewage treatment, calcium hydroxide is used as a clarifying agent or as a flocculant.
- It is used in the paper industry during the Kraft process of converting wood into wood pulp.
- It is a very important compound in the preparation of ammonia.
- This compound is also used as a pH modifier due to its basicity.
Unprotected exposure to Ca(OH)2 can cause severe skin irritation, chemical burns, blindness, lung damage or rashes.