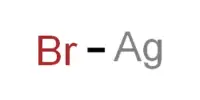Calcium hydride, also known as alkaline earth hydride, is a chemical compound with the formula CaH2. This grey powder (white if pure, which is uncommon) reacts violently with water, releasing hydrogen gas. CaH2 is thus used as a drying agent, also known as a desiccant.
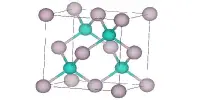
CaH2 is a saline hydride, which means it has a salt-like structure. Saline hydrides are formed by alkali metals and alkaline earth metals heavier than beryllium. Sodium hydride, which crystallizes in the NaCl motif, is a well-known example. These species are insoluble in any solvent that they do not react with. CaH2 crystallizes in the PbCl2 structure (cotunnite).
Properties
The molecular mass of calcium hydride is 42.094 g.mol. Its melting point is 816oC, and its density is 1.9 g.cm-3. As mentioned, whilst being extremely stable it is highly reactive in water and also reacts with alcohols.
- Chemical formula: CaH2
- Molar mass: 42.094 g/mol
- Appearance: gray powder (white when pure)
- Density: 1.70 g/cm3, solid
- Melting point: 816 °C (1,501 °F; 1,089 K)
- Solubility in water: reacts violently
- Solubility: reacts in alcohol
- Crystal structure: Orthorhombic
Preparation
Calcium hydride is made from its elements by combining calcium and hydrogen at temperatures ranging from 300 to 400 °C.
Calcium hydride is easily prepared from its constituent elements by direct combination at high temperatures of 300-4000C. One method of processing is a two-step reduction process that employs vacuum distillation to convert CaO to calcium metal. CaH2 is then formed by heating it in the presence of hydrogen. In the process, metallic sodium can be used.
Uses
This material has a variety of industrial applications. It is commonly used as a desiccant because it is a mild drying agent when compared to more reactive substances such as sodium-potassium alloys. It can be used to dry basic solvents like pyridine and amines.
It is also used in the production of hydrogen, and its use for this purpose dates back to the 1940s, when it was marketed as Hydrolith. It is still used to generate pure hydrogen for research purposes such as fuel cell development. It can also be used to determine the moisture content of diesel.
Safety Issues
Because of its high reactivity when in contact with water, handling calcium hydride requires special precautions. It also reacts violently with other compounds like tetrahydrofuran, potassium perchlorate, chloride, bromate, and hypochlorite. When combined with silver fluoride, it can also become incandescent.
Calcium hydride must be handled in an inert atmosphere due to its reactivity. Because it is irritant and highly corrosive, the material is also extremely hazardous to those who handle it. Lung damage and respiratory inflammation can result from ingestion and inhalation. Blisters and dermatitis can result from skin contact, and eye contact can result in corneal damage and even blindness.