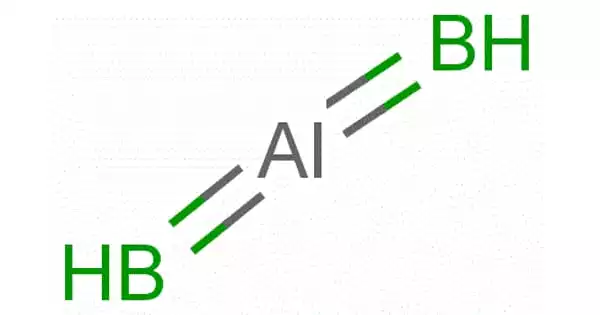
Aluminium diboride (AlB2) is a boron and aluminum chemical combination. It is a chemical compound composed of the metals aluminum and boron. Aluminum is the most abundant metal in the earth’s crust, and it is always found in combination with other elements like oxygen, silicon, and fluorine.
It is one of two compounds of aluminium and boron, the other being AlB1, both of which are known as aluminium boride. The B atoms create graphite-like sheets with Al atoms between them, which is structurally similar to magnesium diboride. Metallic conductivity is seen in single crystals of AlB2 along the axis corresponding to the basal hexagonal plane.
Properties
- Chemical formula: AlB2
- Molar mass: 48.604 g/mol
- Appearance: Copper-red solid
- Density: 3.19 g/cm3
- Melting point: >920 °C (decomposes)
- Solubility in water: insoluble

Aluminium boride is classified as a hazardous material because it produces harmful gases when it combines with acids and hydrogen gas. It, for example, interacts with hydrochloric acid to produce borane and aluminum chloride. Understanding the diverse bonding conditions in distinct metal borides reveals information about their structures and physical properties. Polycrystalline aluminum diboride (AlB2) samples were produced and compared to commercial and literature samples. The relative ease with which boron-rich and aluminum-deficient phases of aluminum borides can be exhibited in AlB2 is one issue that occurred.
AlB2’s crystal structure is frequently utilized as a model structure to characterize intermetallic compounds. The AlB2 structural family has a wide range of structure types.
Aluminum boride has a structure comparable to that of intermetallic compounds, and its structure is determined mostly by the crystal structure of aluminum metal and boron rather than their valence connection. Aluminum borides are classified as AlB2, AlB4, and AlB12. The interaction of two elemental compounds at temperatures exceeding 600°C can produce the diboride AlB2.
Aluminum boride (AlB2) is a binary chemical produced from the elements aluminum and boron. Under typical conditions of temperature and pressure, it is a red solid. When heated, it loses its surface sheen. It is stable in cold dilute acid but decomposes in hot hydrochloric and nitric acids. It is made by heating and reacting tiny powders of aluminum and boron. It is one of two aluminum-boron compounds, the other being AlB12, that are generally referred to as aluminum boride.