Barium ferrite is a chemical compound with the formula BaFe12O19. This and related ferrite materials are used in magnetic stripe cards and loudspeaker magnets. The Fe3+ centers are ferromagnetically coupled. This field of technology is commonly regarded as an application of the related fields of materials science and solid state chemistry.
Barium ferrite is a metal oxide with a high packing density. This material has been studied since at least 1931 and has found applications in magnetic card strips, speakers, and magnetic tapes. It has found particular success in long-term data storage; the material is magnetic, resistant to temperature change, corrosion, and oxidization.
Properties
Barium ferrite has been considered for long-term data storage. The material has proven to be resistant to a variety of environmental stresses, including humidity and corrosion. Because ferrites have already been oxidized, they cannot be oxidized further. This is one of the reasons ferrites are so resistant to corrosion. Barium ferrite also demonstrated resistance to thermal demagnetization, another issue associated with long-term storage. Curie temperature is typically around 450 C. (723 K).
- Chemical formula: BaFe12O19
- Molar mass: 1111.448 g·mol−1
- Appearance: black solid
- Density: 5.28 g/cm3
- Melting point: 1,316 °C (2,401 °F; 1,589 K)
- Solubility in water: insoluble

When the temperature of barium ferrite magnets rises, their high intrinsic coercivity improves, making them more resistant to thermal demagnetization. Ferrite magnets are the only magnets that become significantly more resistant to demagnetization as the temperature rises. This property of barium ferrite makes it a popular material for motor and generator designs, as well as loudspeaker applications. Ferrite magnets can be used in temperatures as high as 300 °C, making them ideal for the applications listed above. Ferrite magnets are excellent insulators, allowing no electrical current to pass through them, and are brittle, demonstrating their ceramic properties. Ferrite magnets also have good machining properties, which allows for the material to be cut in many shapes and sizes.
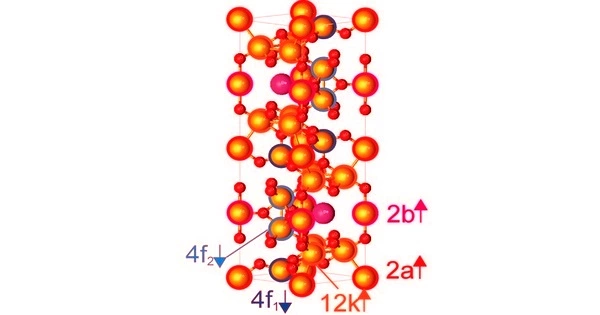
Chemical structure
The Fe3+ centers are ferromagnetically coupled and have a high-spin d5 configuration. This field of technology is commonly regarded as an application of the related fields of materials science and solid-state chemistry.
A related family of industrially useful “hexagonal ferrites” containing barium is known. In contrast to the usual spinel structure, these materials have a hexagonal close-packed oxide framework. Furthermore, some of the oxygen centers are replaced by Ba2+ ions. These species’ formulas include BaFe12O19, BaFe15O23, and BaFe18O27.
To form crystals of barium ferrite, a one-step hydrothermal process involving the mixing of barium chloride, ferrous chloride, potassium nitrate, and sodium hydroxide with a hydroxide to chloride concentration ratio of 2:1 can be used. Ferric nitrate, barium chloride, sodium citrate, and sodium hydroxide are used to make nanoparticles. However, the most common method of preparation is to calcine barium carbonate with iron(III) oxide:
BaCO3 + 6 Fe2O3 Δ→ BaFe12O19 + CO2
Chemical properties
Barium ferrites are robust ceramics that are generally stable to moisture and corrosion-resistant. BaFe is also an oxide so it does not break down due to oxidation as much as a metal alloy might; giving BaFe a much greater life expectancy.
Mechanical properties
Metal particles (MP) have been used to store data on tapes and magnetic strips, but they have reached their capacity limit for high-capacity data storage. To increase their capacity on data tape by (25x), the MP had to increase the tape length by (45%) and track density by over (500%), which necessitated shrinking the individual particles. As the particle size was reduced, the passivizing coating required to prevent oxidation and deterioration of the MP had to become thicker. This posed a problem because as the passivation coating thickened, it became more difficult to achieve an acceptable signal-to-noise ratio.
Applications
In applications such as recording media, permanent magnets, and magnetic stripe cards, barium ferrite is used (credit cards, hotel keys, ID cards). Because of the material’s stability, it can be greatly reduced in size, resulting in a much higher packing density. Earlier media devices used doped acicular oxide materials to generate the coercivity values required for recording. Barium ferrite has largely replaced acicular oxides in recent decades; without dopants, acicular oxides produce very low coercivity values, making the material magnetically soft, whereas barium ferrite’s higher coercivity levels make the material magnetically hard, making it a superior choice for recording material applications.