Silver is a metallic element that typically loses one electron to become a positively charged ion, Ag+1. The sulfur atom needs two electrons to fill its valence electron shell. A valence electron shell is the outermost part of an atom’s electron cloud. When a sulfur atom gains two electrons, it becomes a negatively charged ion, S+2. Sulfur can get these two electrons from two silver atoms.
Silver sulfide (Ag2S) is a dense black solid that is insoluble in all solvents but is degraded by strong acids. It is the sulfide of silver. When silver sulfide is formed on electrical contacts operating in an atmosphere rich in hydrogen sulfide, it results in the formation of long filaments known as silver whiskers. It is found in nature as a relatively low-temperature mineral acanthite. It is useful as a photosensitizer in photography.
The air contains small traces of hydrogen sulfide gas from rotting plants and animal carcasses. Since silverware is used to eat food, it makes sense that foods containing sulfur can cause tarnishing if the silverware isn’t cleaned properly. Common foods containing sulfur are eggs, mayonnaise, and mustard.

Structure and Properties of Silver Sulfide
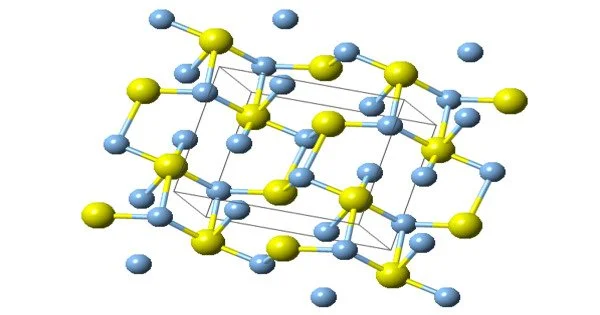
Chemical deposition from aqueous solutions of silver nitrate and sodium sulfide was used for the synthesis of coarse-crystalline and nanocrystalline silver-sulfide Ag2S powders. There are three forms of structures, they are known: monoclinic acanthite (β-form), stable below 179 °C, body-centered cubic so-called argentite (α-form), stable above 180 °C, and a high-temperature face-centered cubic (γ-form) stable above 586 °C. The higher temperature forms are electrical conductors. It is found in nature as relatively low-temperature mineral acanthite. Acanthite is an important ore of silver. In the acanthite, monoclinic, form there are two crystallographically distinct silver atoms with two and three near neighbor sulfur atoms respectively. The name argentite refers to a cubic form, which, due to instability in “normal” temperatures, is found in form of the pseudo-morphosis of acanthite after argentite.
The unit cell of artificial monoclinic silver sulfide Ag2S contains four Ag2S formula units and has the following parameters: a = 0.42264 nm, b = 0.69282 nm, c = 0.95317 nm, and β = 125.554°.
Silver sulfide is insoluble in all solvents, but is degraded by strong acids. Silver sulfide is a network solid made up of silver (electronegativity of 1.98) and sulfur (electronegativity of 2.58) where the bonds have low ionic character (approximately 10%). It is a component of the classical qualitative inorganic analysis. When formed on electrical contacts operating in an atmosphere rich in hydrogen sulfide, long filaments known as silver whiskers can form. When combined with silver the hydrogen sulfide gas creates a layer of black silver sulfide patina on the silver, protecting the inner silver from further conversion to silver sulfide.