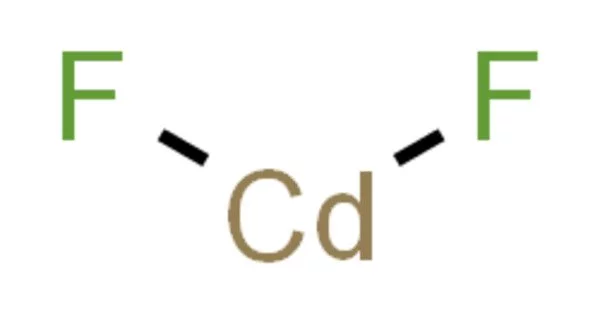
Cadmium fluoride (CdF2) is a mostly water-insoluble source of cadmium used in oxygen-sensitive applications such as the production of metallic alloys. This and other fluoride compounds are used in very low concentrations (ppm) in a few medical treatment protocols. Fluoride compounds are also important in synthetic organic chemistry. The standard enthalpy was determined to be -167.39 kcal. mole-1, the Gibbs energy of formation was determined to be -155.4 kcal. mole-1, and the heat of sublimation was determined to be 76 kcal. mole-1.
Cadmium fluoride is used in the production of phosphors, glass, and nuclear reactor controls. It is also used in high-temperature lubricants and to ignite laser crystals. It is used in oxygen-sensitive applications such as the manufacture of metallic alloys, as well as in a few medical treatment protocols. It can also be used in synthetic organic chemistry.
Properties
- Chemical formula: CdF2
- Molar mass: 150.41 g/mol
- Appearance: grey or white-grey crystals
- Density: 6.33 g/cm3, solid
- Melting point: 1,110 °C (2,030 °F; 1,380 K)
- Boiling point: 1,748 °C (3,178 °F; 2,021 K)
- Solubility in water: 4.35 g/100 mL
- Solubility: soluble in acid, insoluble in ethanol alcohol, and liquid ammonia
- Crystal structure: Fluorite (cubic), cF12

Preparation
Cadmium fluoride is made by reacting gaseous fluorine or hydrogen fluoride with cadmium metal or one of its salts, such as chloride, oxide, or sulfate. It can also be made by dissolving cadmium carbonate in 40% hydrofluoric acid, evaporating the solution, and drying it in a vacuum at 150 °C. Another method is to combine cadmium chloride and ammonium fluoride solutions, then crystallize them. The solution is filtered to remove the insoluble cadmium fluoride.
Adium fluoride is made by reacting gaseous fluorine or hydrogen fluoride with cadmium metal or a salt of cadmium metal, such as chloride, oxide, or sulfide:
Cd + F2 → CdF2
Cd + 2HF → CdF2 + H2
CdO + 2HF → CdF2 + H2O
It also may be obtained by dissolving cadmium carbonate in 40% hydrofluoric acid solution, evaporating the solution and drying in vacuum at 150°C:
CdCO3 + 2HF → CdF2 + H2O + CO2
It can also be made by combining cadmium chloride and ammonium fluoride solutions and crystallizing them. Cadmium fluoride has also been synthesized by combining fluorine and cadmium sulfide. This reaction occurs quickly and produces nearly pure fluoride at much lower temperatures than other methods.
Uses
When doped with certain rare earth elements or yttrium and treated with cadmium vapor at high temperatures, CdF2 can be transformed into an electronic conductor. Depending on the concentration of the dopant, this process produces blue crystals with varying absorption coefficients.
A proposed mechanism explains that the conductivity of these crystals can be explained by a reaction of Cd atoms with Interstitial F− ions. This creates more CdF2 molecules and releases electrons which are weakly bonded to trivalent dopant ions resulting in n-type conductivity and a hydrogenic donor level.
Safety
Cadmium fluoride is toxic, as are all cadmium compounds, and should be used with caution.
Cadmium fluoride can be harmful to one’s health if not handled properly. Because it can irritate the skin and eyes, gloves and protective eyewear are recommended. The MSDS, or Material Safety Data Sheet, also includes ingestion and inhalation warnings. Hydrogen fluoride and cadmium vapors may be released into the air under acidic conditions, at high temperatures, and in moist environments. Inhalation can cause respiratory irritation, congestion, fluorosis, and even pulmonary edema in severe cases.