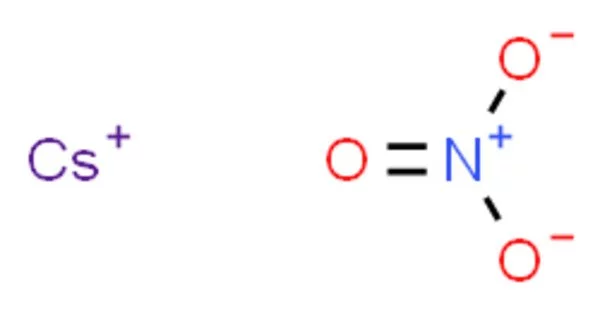Caesium nitrate, also known as cesium nitrate, is a salt with the chemical formula CsNO3. It is a strong oxidizer that can catch fire when it comes into contact with organic materials. It is an alkali metal nitrate that is used in pyrotechnic compositions as a colorant and oxidizer, for example, in decoys and illumination flares. The caesium emissions are primarily caused by two powerful spectral lines at 852.113 nm and 894.347 nm.
Cesium nitrate is used as a colorant and oxidizer in the pyrotechnic industry, petroleum cracking, scintillation counters, and x-ray phosphors. It is used as a promoter in heterogeneous catalysts.
Properties
Cesium nitrate is a colorless, glittering crystalline solid. It is soluble in water. It is a strong oxidizing material and may burst into flames on contact with organic materials.
- Chemical formula: CsNO3
- Molar mass: 194.91 g/mol
- Appearance: white solid
- Density: 3.685 g/cm3
- Melting point: 414 °C (777 °F; 687 K)
- Boiling point: decomposes, see text
- Solubility in water: 9.16 g/100 ml (0 °C); 196.8 g/100 ml (100 °C)
- Solubility in acetone: soluble
- Solubility in ethanol: slightly soluble

Applications
Caesium nitrate prisms are used in infrared spectroscopy, in x-ray phosphors, and in scintillation counters. It is also used in making optical glasses and lenses.
As with other alkali metal nitrates, caesium nitrate decomposes on gentle heating to give caesium nitrite: 2 CsNO3 → 2 CsNO2 + O2
Caesium also forms two unusual acid nitrates, which can be described as CsNO3·HNO3 and CsNO3·2HNO3 (melting points 100 °C and 36–38 °C respectively).
Cesium nitrate prisms are used in infrared spectroscopy, x-ray phosphors, and scintillation counters. It is a precursor material for the synthesis of cesium nitratocuprate and cesium salts. Cesium nitrate doped on carbon-based ruthenium catalyst plays an important role in the synthesis of ammonia.
Health Hazard
Inhalation, ingestion, or skin or eye contact with vapors or substances can result in severe injury, burns, or death. Fire can emit irritant, corrosive, and/or toxic gases. Pollution may result from runoff from firefighting or dilution water.
When these substances are involved in a fire, they will accelerate the burning process. When heated or involved in a fire, some may decompose explosively. Heat or contamination may cause an explosion. Some will explode when combined with hydrocarbons (fuels). Combustibles may be ignited (wood, paper, oil, clothing, etc.). When heated, containers may explode. Runoff can cause a fire or explosion.