Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite and three considerably rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, which means it possesses both basic and acidic characteristics. Aluminium oxide hydroxide, AlO(OH), and amphoteric aluminium oxide or alumina (Al2O3) are closely related. These compounds are the primary constituents of the aluminium ore bauxite.
Properties
Amorphous powder-white aluminic hydroxide It is insoluble in water but soluble in alkaline and acidic solutions. It can be found in nature as the mineral gibbsite and its polymorphs doyleite, nordstrandite, and bayerite.
- Chemical formula: Al(OH)3
- Molar mass: 78.00 g/mol
- Appearance: White amorphous powder
- Density: 2.42 g/cm3, solid
- Melting point: 300 °C (572 °F; 573 K)
- Solubility in water: 0.0001 g/100 mL
- Solubility: Soluble in acids and alkalis
- Isoelectric point: 7.7
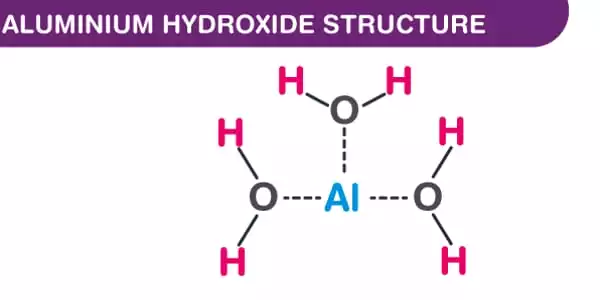
Structure
Al(OH)3 is composed of two layers of hydroxyl groups, with aluminum ions filling two-thirds of the octahedral gaps between the layers. There are four distinct polymorphs. Layers of octahedral aluminium hydroxide units are present in all of them, with hydrogen bonds connecting them. In terms of layer stacking, the polymorphs differ. All Al(OH)3 crystal formations are hexagonal:
- gibbsite is also known as γ-Al(OH)3 or α-Al(OH)3
- bayerite is also known as α-Al(OH)3 or β-alumina trihydrate
- nordstrandite is also known as Al(OH)3
- doyleite
Hydrargillite, once thought to be aluminium hydroxide, is aluminium phosphate. Nonetheless, both gibbsite and hydrargillite refer to the same polymorphism of aluminium hydroxide, with gibbsite used most commonly in the United States and hydrargillite used more often in Europe. Hydrargillite is named after the Greek words for water (hydra) and clay (argylles).
Aluminium hydroxide is amphoteric. In acid, it acts as a Brønsted–Lowry base. It neutralizes the acid, yielding a salt:
3 HCl + Al(OH)3 → AlCl3 + 3 H2O
In bases, it acts as a Lewis acid by binding hydroxide ions:
Al(OH)3 + OH− → Al(OH)4−
Production
The Bayer method produces commercially usable aluminium hydroxide. It is accomplished by dissolving bauxite in sodium hydroxide solution at temperatures ranging from 0 to 270 °C. After removing the trash, the sodium aluminate solution is allowed to precipitate. As a result, the precipitate formed is aluminium hydroxide. Calcination can be used to produce alumina or aluminium oxide from aluminium hydroxide.
The Bayer method [which includes dissolving bauxite in sodium hydroxide at temperatures up to 270 °C (518 °F)] produces nearly all of the aluminium hydroxide used commercially. The waste solid, bauxite tailings, is removed, and aluminium hydroxide is precipitated from the sodium aluminate solution that remains. Calcination can transform this aluminum hydroxide to aluminum oxide or alumina.
Because of the leftover sodium hydroxide, the residue or bauxite tailings, which is primarily iron oxide, is highly caustic. It was formerly stored in lagoons, which resulted in the 2010 Ajka alumina factory tragedy in Hungary, in which a dam burst, drowning nine people. Another 122 people required treatment for chemical burns. The dirt contaminated 40 square kilometres (15 square miles) of land and eventually made its way to the Danube. While the mud was deemed non-toxic due to low heavy metal levels, the related slurry had a pH of 13.
Uses
- Aluminium hydroxide is used as a flame retardant in plastics.
- Used as an antacid.
- Used in aluminium Hydroxide gel.
- Used to manufacture activated alumina.
- Used as a filler in cosmetics.
- Used as a chemical intermediate.
- Used as a soft abrasive for plastics.
- Used in glass additive to increase resistance to thermal shock.
- Used in waterproofing fabrics.
- Used in the manufacturing of glass.
Safety
Prolonged exposure to Aluminium(III) hydroxide it causes irritation in eyes, respiratory system, and skin. When comes in contact with water it causes a violent explosion.