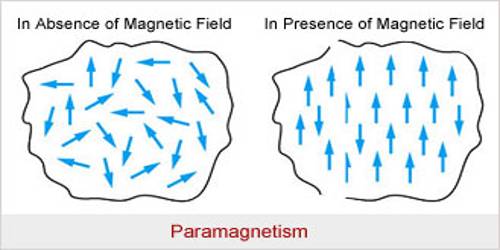
Paramagnetic Substance
Atoms, molecules or ions in the paramagnetic materials contain permanent magnetic moments. Due to the combined effect of the orbital moment and spin moment of the electrons permanent moment of the atoms and ions is created in these materials. In short, it can be said that the materials which acquire a small amount of magnetism towards the magnetic field when they are placed in a magnetic field are called paramagnetic material. Example – sodium, antimony, platinum, manganese, liquid oxygen, chromium etc.
- They are feebly attracted by magnets.
- They can be solids, liquids, and gases.
- They have no retentively.
- They have no curie points.
- Magnetic susceptibility is positive but of low value.
- They do not have hysteresis properties.
- Magnetic permeability is of low order, µ > 1.
- Their susceptibility depends on temperature i.e., K ∞ 1/T.
- Magnetism disappears after removal of the magnetic field.
- If placed in magnetic field, they tend to go to the stronger region of the field from weaker region.