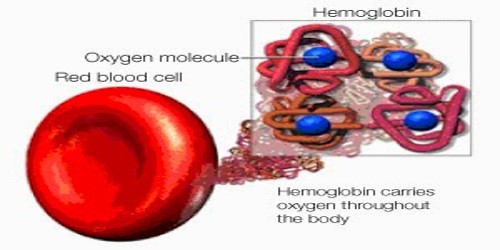
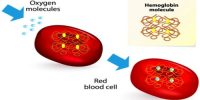
Hemoglobin (Hb) may be defined as a vital conjugated protein present inside the Red Blood Cells (RBC). It is the protein molecule in red blood cells that bear oxygen from the lungs to the body’s tissues and returns carbon dioxide from the tissues back to the lungs. Hemoglobin is made up of four subunits and can bind up to four oxygen molecules.
Iron is in ferrous (Fe++) form in heam and exists as 6 covalent states. By its four co-ordinate valency, it remains attached to N of the 4 pyrrole rings, and by its 5th valency to the N of the imidazole group in the associated globin, Only one co-ordinate valency is left. Oxygen combines loosely with this co-valency iron atom in the form of Hb oxygen. The majority of oxygen in the body is transported by hemoglobin, which is found in red blood cells.
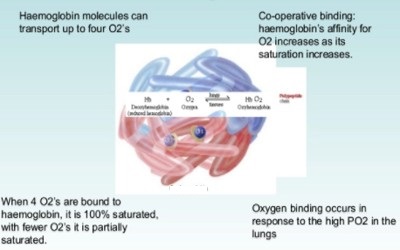
Fig: Molecule of Hemoglobin (Hb) carry four molecules of Oxygen
Hemoglobin is a protein molecule found in red blood cells made of four subunits: two alpha subunits and two beta subunits. Each subunit surrounds a central heme group that contains iron and binds one oxygen molecule, allowing each hemoglobin molecule to bind four oxygen molecules. So one molecule of Fe carries one molecule of oxygen. Now 1 molecular Hb carries 4 atoms of Fe. Thus one molecule of Hb carries 4 molecules of oxygen.