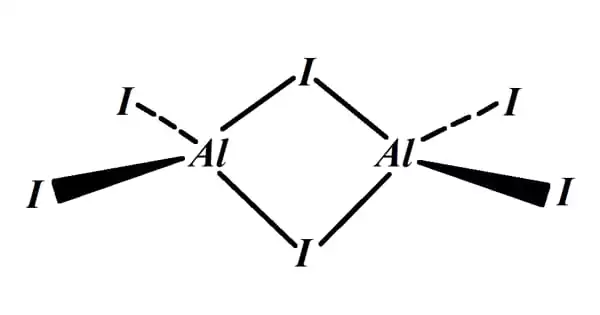
Aluminium iodide is a chemical compound that contains both aluminum and iodine. It is created through the reaction of aluminum with iodine, which is the reaction of aluminum hydroxide with hydroiodic acid. The name invariably refers to an AlI3 combination generated by the reaction of aluminium and iodine or the action of HI on Al metal.
It is a binary compound group of inorganic Lewis acid, a specific sort of chemical capable of receiving an electron pair. Aluminum iodide, like other types of aluminum compounds, may absorb water from the air. It is frequently referred to as aluminum salt or hydroiodic acid.
Properties
Aluminum iodide is white with a yellowish hue. Along with dissolving in water, aluminum iodide can be absorbed by alcohol, ether, carbon sulfide, and pyridine, a colorless liquid from coal tar. It is a colorless powder that has a density of 3.98 g/cm3. Furthermore, its melting point is 189.4oC and the boiling point is 360oC. It has a molar mass of 407.69 g/mol.
- Molecular Weight: 407.7
- Appearance: Tan to reddish-brown powder
- Melting Point: 189.4° C (372.9° F)
- Boiling Point: 360° C (680° F)
- Density: 3.98 g/cm3
- Solubility in H2O: N/A

Preparation
Iodine belongs to the halogen family which is highly reactive non-metals. Also, it is a diatomic element which means it is highly reactive and is much more stable in molecular form. That gives I2. Besides, aluminum exists in an atomic form so the reaction between aluminum and iodine is:
Al + I2 → AlI3
The process begins with two atoms of iodine and concludes with three atoms of iodine. This occurs because an aluminum atom must lose one electron or gain three electrons to complete its outer shell electrons. Similarly, in order to complete its outer shell, iodine must either gain or lose one electron. As a result, aluminum requires three iodine atoms to complete its outer shell.
The hexahydrate is formed by reacting metallic aluminum or aluminum hydroxide with hydrogen iodide or hydroiodic acid. AlI3, like the related chloride and bromide, is a strong Lewis acid that will take water from the atmosphere. It is used as a reagent for the scission of specific types of C-O and N-O bonds. It cleaves aryl ethers and deoxygenates epoxides.
Uses
Aluminum iodide has several applications, including veterinary medicine and pigs. When pigs get pneumonia, they are sprayed with an aerosol version of aluminum iodide. It is also used to kill pathogens and clean horse and cattle barns. Spraying it in regions where calves and pigs are present helps protect them from respiratory infections.
Aluminum iodide has a lot of antibacterial properties, although it has little effect on mucous membranes. New applications are being investigated in order to improve pathogenic and therapeutic effects.