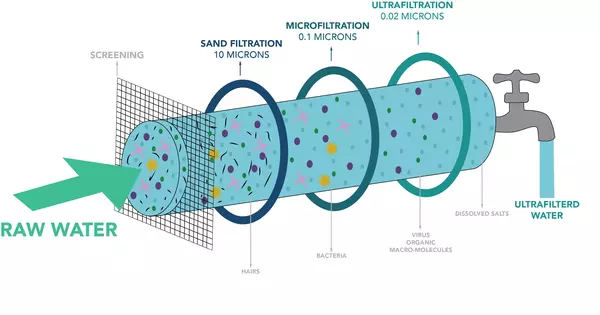
Ultrafiltration (UF) is a liquid separation technique that employs a semi-permeable membrane to remove suspended or dissolved solids. It’s a type of membrane filtration in which forces like pressure or concentration gradients cause separation through a semipermeable membrane. In terms of pore size, it is a pressure-driven membrane filtration technique that falls between microfiltration and nanofiltration.
Water and low molecular weight solutes pass through the membrane in the permeate (filtrate), while suspended solids and high molecular weight solutes are retained in the retentate. In industry and research, this separation process is used to purify and concentrate macromolecular (103-106 Da) solutions, particularly protein solutions.
Key points about ultrafiltration:
- Membrane Pore Size: The pores in ultrafiltration membranes are typically between 0.1 and 0.001 micrometers in size. Water and small solutes can pass through these pores, but larger particles such as suspended solids, colloids, bacteria, and macromolecules are blocked.
- Principle: Size exclusion is the basis for separation in ultrafiltration. The process is usually propelled by hydraulic pressure, which forces the liquid through the membrane and separates the components based on their size.
Applications:
- Water Treatment: UF is commonly used in water and wastewater treatment to remove particles, bacteria, and other impurities.
- Food and Beverage Industry: UF is employed in processes such as dairy processing, juice clarification, and protein concentration.
- Biopharmaceuticals: Ultrafiltration is used in the pharmaceutical industry for the concentration and purification of proteins and other biomolecules.
- Industrial Processes: It finds applications in various industrial processes for the separation and concentration of specific components from liquids.
Advantages:
- Efficient removal of particles and solutes based on size.
- Relatively gentle process, preserving the integrity of sensitive molecules.
- Can be used for the concentration and purification of various substances.
Limitations:
- Fouling: Membrane fouling can occur, reducing efficiency over time.
- Operating Costs: The process may require higher operating pressures, leading to increased energy costs.
- Sensitivity: Membranes can be sensitive to certain chemicals and extreme pH conditions.
- Module Configuration: UF systems are often configured as spiral-wound modules or tubular modules, depending on the specific application and operational requirements.
Ultrafiltration is not fundamentally different from microfiltration. Both of these are distinct based on size exclusion or particle capture. It is fundamentally different from membrane gas separation, which separates based on different amounts of absorption and different rates of diffusion. The molecular weight cut-off (MWCO) of the membrane is used to define ultrafiltration membranes. Ultrafiltration is used in cross-flow or dead-end mode.
Ultrafiltration is important in many industries where the separation and concentration of specific components from liquids is required. Its application is expanding as technology and membrane materials improve.