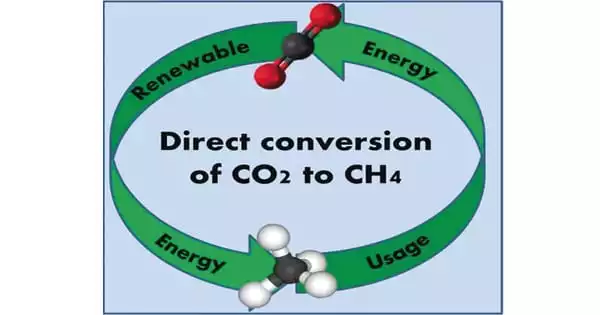
Researchers can produce methane from captured CO2 and renewable hydrogen, paving the way for cheaper synthetic natural gas. Researchers at the Department of Energy’s Pacific Northwest National Laboratory have developed a method to convert captured carbon dioxide (CO2) into methane, the primary component of natural gas, as part of their ongoing effort to make carbon capture more affordable.
While anthropogenic CO2 emissions continue to rise, recycling carbon dioxide (CO2), particularly through conversion to methane (CH4), is compelling. Photothermal methanation is a useful process for this transformation, in which CO2 and hydrogen are catalytically converted into CH4 and water when exposed to sunlight. A team of researchers has now reported the synthesis of a highly active, stable nickel-carbon catalyst for this reaction in the journal Angewandte Chemie.
The researchers’ new method reduces the materials required to run the reaction, the energy required to fuel it, and, ultimately, the selling price of the gas by streamlining a long-standing process in which CO2 is converted to methane.
Methane can be produced from renewable or recycled CO2 sources, in addition to geologic sources, and can be used as a fuel or as an H2 energy carrier. Methane, despite being a greenhouse gas that requires careful supply chain management, has many applications ranging from household use to industrial processes.
Jotheeswari Kothandaraman
The team at King Abdullah University of Science and Technology (Thuwal, Saudi Arabia) led by Luis Garzón-Tovar and Jorge Gascon was looking for an efficient, cost-effective catalyst for photothermal methanation of CO2. The combination of light-driven and thermal chemical processes underpins photothermal catalysis. Unlike pure photocatalysis, it allows longer wavelength light in the visible and infrared regions of the spectrum to contribute to driving the reaction.
Instead of precious metals, they chose a high load of nickel nanoparticles on carbon-based support to base the new catalyst on an abundant, inexpensive metal. Carbon materials are highly promising photothermal catalysis supports because they absorb a wide range of light, are highly efficient at converting light to heat energy, and have a large surface area.
The catalyst was created using a nickel-containing metal-organic framework (Ni-MOF-74) as the starting material. This material performed best when pyrolyzed under controlled conditions at 600°C. When Ni-MOF-74 decomposes, it leaves behind uniformly finely distributed nickel nanoparticles embedded in a porous graphitic carbon matrix. Under artificial UV, visible, and infrared light, the resulting material, dubbed Ni@C, demonstrated a high rate of conversion and high selectivity for methanation. The efficiency of the catalyst remained stable over a period of more than 12 hours in a continuous process in a flow-type reactor.
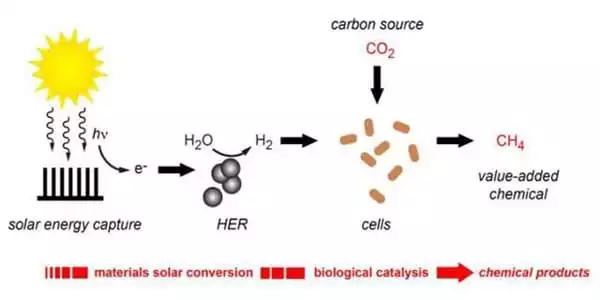
An experiment was conducted outside, under natural sunlight, to demonstrate the system’s practical application, demonstrating the potential of this new catalyst to reduce CO2 to CH4 using solar energy.
Methane’s role in carbon capture
Various methods for converting CO2 to methane have been known for a long time. Most processes, however, rely on high temperatures and are frequently too expensive for widespread commercial use.
Methane can be produced from renewable or recycled CO2 sources, in addition to geologic sources, and can be used as a fuel or as an H2 energy carrier. Methane, despite being a greenhouse gas that requires careful supply chain management, has many applications ranging from household use to industrial processes, according to lead author and PNNL chemist Jotheeswari Kothandaraman.
“Right now, a large portion of the natural gas used in the United States must be pumped from the ground,” Kothandaraman explained, “and demand is expected to rise over time, even under climate change mitigation pathways.” The methane produced by this process, which uses waste CO2 and renewable hydrogen, could provide an alternative for utilities and consumers seeking natural gas with a renewable component and a lower carbon footprint.”
Making more from CO2
According to Kothandaraman, the chemical process highlighted in the paper is just one of many where captured CO2 can be used as a feedstock to produce other valuable chemicals.
“I’ll be happy when I can get this process to work for methanol as well as it does for methane,” she said. “That’s my long-term ambition.” Methanol has far more applications than methane, according to Kothandaraman, who has spent the last decade researching the catalytic reactions that could produce methanol from CO2. Another avenue the team intends to pursue is the production of plastics from captured CO2.
“It’s critical that we not only capture CO2, but also find valuable uses for it,” said Ron Kent, Advanced Technologies Development Manager at SoCalGas, “and this study provides a cost-effective pathway toward making something valuable out of waste CO2.”