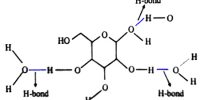
The honey is a mixture of water and glucose.
The atomic mass of glucose (C6 H12 O6)
= (6 x 12) + (12 x 1) + (6 x 16) = 72 + 12 + 96 = 180
According to the stimulus,
In 100 gm honey, the mass of glucose is = (100 x 0.8) g = 80 g
The mole number of glucose = 80/180 = 4/9
Again, The mass of water = (100 x 0.2) g = 20g
Hence, the mole number of H2O =20/18 = 10/9
The ratio is (10/9) / (4/9) = 2.5 moles
So, 2.5 moles of water are present is per glucose molecule in honey.