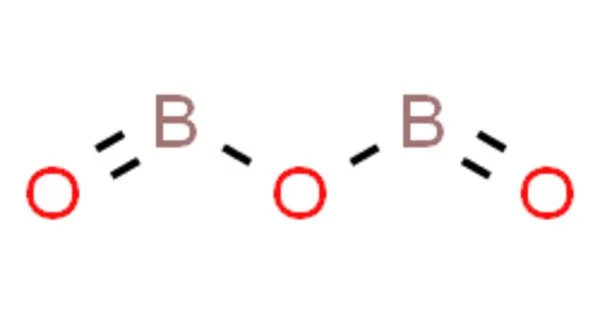
Boron trioxide, also known as diboron trioxide, is a boron oxide with the formula B2O3. It is a white solid that is an inorganic polymer that is used in the production of glass and enamel as well as the synthesis of other boron compounds. It is a colorless, transparent solid that is almost always glassy (amorphous) and can only be crystallized with extreme difficulty. Boria is another name for boric oxide. It has numerous important industrial applications, most notably in ceramics as a flux for glazes and enamels and in glass production.
Properties
It is white, glassy, and solid. It is almost always found as the vitreose (amorphic) form; however, it can be crystallized after extensive annealing. It is one of the most difficult compounds known to crystallize.
- Chemical formula: B2O3
- Molar mass: 69.6182 g/mol
- Appearance: white, glassy solid
- Density: 2.460 g/cm3, liquid; 2.55 g/cm3, trigonal; 3.11–3.146 g/cm3, monoclinic
- Melting point: 450 °C (842 °F; 723 K) (trigonal); 510 °C (tetrahedral)
- Boiling point: 1,860 °C (3,380 °F; 2,130 K), sublimes at 1500 °C
- Solubility in water: 1.1 g/100mL (10 °C); 3.3 g/100mL (20 °C); 15.7 g/100mL (100 °C)
- Solubility: partially soluble in methanol

Preparation
Boron trioxide is created in a fusion furnace by reacting borax with sulfuric acid. The molten boron oxide layer separates from sodium sulfate at temperatures above 750°C. After that, it is decanted, cooled, and purified to 96-97% purity.
Heating boric acid above 300°C is another method. At around 170°C, boric acid decomposes into steam, (H2O(g)), and metaboric acid (HBO2), and further heating above 300°C produces more steam and diboron trioxide. The reactions are as follows:
H3BO3 → HBO2 + H2O
2 HBO2 → B2O3 + H2O
Boric acid goes to anhydrous microcrystalline B2O3 in a heated fluidized bed. Carefully controlled heating rate avoids gumming as water evolves.
Boron oxide will also form when diborane (B2H6) reacts with oxygen in the air or trace amounts of moisture:
2B2H6(g) + 3O2(g) → 2B2O3(s) + 6H2(g)
B2H6(g) + 3H2O(g) → B2O3(s) + 6H2(g)
Structure
Boron trioxide has three known forms, one amorphous and two crystalline.
Reactions
Molten boron oxide attacks silicates. Containers can be passivated internally with a graphitized carbon layer obtained by thermal decomposition of acetylene.
Amorphous form
By far the most common is the amorphous form (g-B2O3). It is thought to be made up of boroxol rings, which are six-membered rings made up of alternating three-coordinate boron and two-coordinate oxygen.
This viewpoint was initially controversial due to the difficulty of building disordered models at the correct density with many boroxol rings, but such models have recently been constructed and exhibit properties that are in excellent agreement with experiment. Experimental and theoretical studies have revealed that the fraction of boron atoms belonging to boroxol rings in glassy B2O3 ranges between 0.73 and 0.83, with 0.75 = 3/4 corresponding to a 1:1 ratio between ring and non-ring units. In the liquid state, the number of boroxol rings decreases as temperature rises.
Applications
- Major component of borosilicate glass
- Fluxing agent for glass and enamels
- Starting material for synthesizing other boron compounds such as boron carbide
- An additive used in glass fibres (optical fibres)
- The inert capping layer in the Liquid Encapsulation Czochralski process for the production of gallium arsenide single crystal
- As an acid catalyst in organic synthesis.