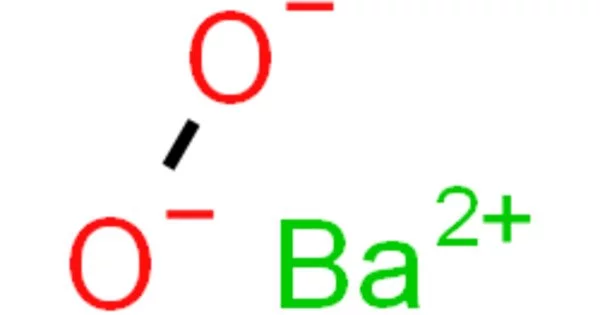The inorganic compound with the formula BaO2 is barium peroxide. It is a compound that is produced as a grayish white toxic powder by heating barium monoxide in air or oxygen and is primarily used in the production of hydrogen peroxide and in pyrotechnics. This white solid (gray when impure) is the first peroxide compound discovered and is one of the most common inorganic peroxides. Because it is an oxidizer and emits a bright green flame when ignited (as do all barium compounds), it is used in fireworks; historically, it was also used as a precursor for hydrogen peroxide.
It is a grayish-white dry powder that can be stored in paper packages and is an excellent bleaching agent. When mixed with water, it releases its bleaching properties.
Properties
Barium peroxide is a grayish-white powder with a slight water solubility. When it comes into contact with organic materials, it causes a dangerous fire and explosion risk and decomposes at around 1450°F (787°C). It is also toxic by ingestion, a skin irritant, and should be stored cool and dry.
- Chemical formula: BaO2
- Molar mass: 169.33 g/mol (anhydrous); 313.45 (octahydrate)
- Appearance: Grey-white crystalline (anhydrous); colorless solid (octahydrate)
- Odor: odorless
- Density: 5.68 g/cm3 (anhydrous) 2.292 g/cm3 (octahydrate)
- Melting point: 450 °C (842 °F; 723 K)
- Boiling point: 800 °C (1,470 °F; 1,070 K) (decomposes to BaO & O2)
- Solubility in water: anhydrous 0.091 g/100 mL (20 °C); octahydrate 0.168 g/cm3
- Solubility: dissolves with decomposition in acid

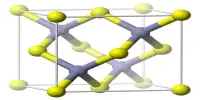
Structure
Barium peroxide is a peroxide, containing O22- subunits. The solid is isomorphous to calcium carbide, CaC2.
Preparation and use
Barium peroxide arises by the reversible reaction of O2 with barium oxide. The peroxide forms around 500 °C and oxygen is released above 820 °C.
2 BaO + O2 ⇌ 2 BaO2
This reaction is the basis for the now-obsolete Brin process for separating oxygen from the atmosphere. Other oxides, e.g. Na2O and SrO, behave similarly.
In another obsolete application, barium peroxide was once used to produce hydrogen peroxide via its reaction with sulfuric acid:
BaO2 + H2SO4 → H2O2 + BaSO4
The mixture is filtered to remove the insoluble barium sulfate. It slowly decomposes in air, releasing hydroxide and oxygen. It does not dissolve in water, but it can hydrolyze slowly, producing hydrogen peroxide in solution. It is a strong oxidizing agent that will explode if it comes into contact with organic matter.
Applications
One of the most common inorganic peroxides is barium peroxide. It is used as an oxidizer in a variety of military and pyrotechnic mixtures. It is also used to bleach animal and vegetable fibers, as well as straw. It is also used in the production of oxygen and hydrogen peroxide. It is used in the welding industry when combined with aluminum. It can be used in organic synthesis as an oxidizer.
Health Hazards
Inhaling barium peroxide irritates the mucous membranes, throat, and nose. Severe burns result from contact with the eyes or skin. Severe salivation, vomiting, colic, diarrhea, convulsive tremors, slow, hard pulse, and elevated blood pressure result from ingestion.